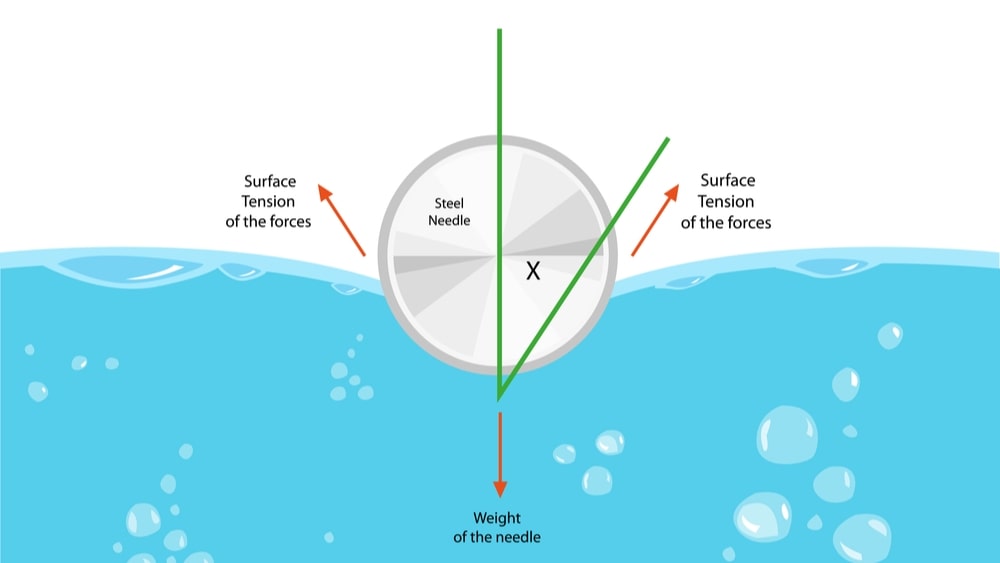
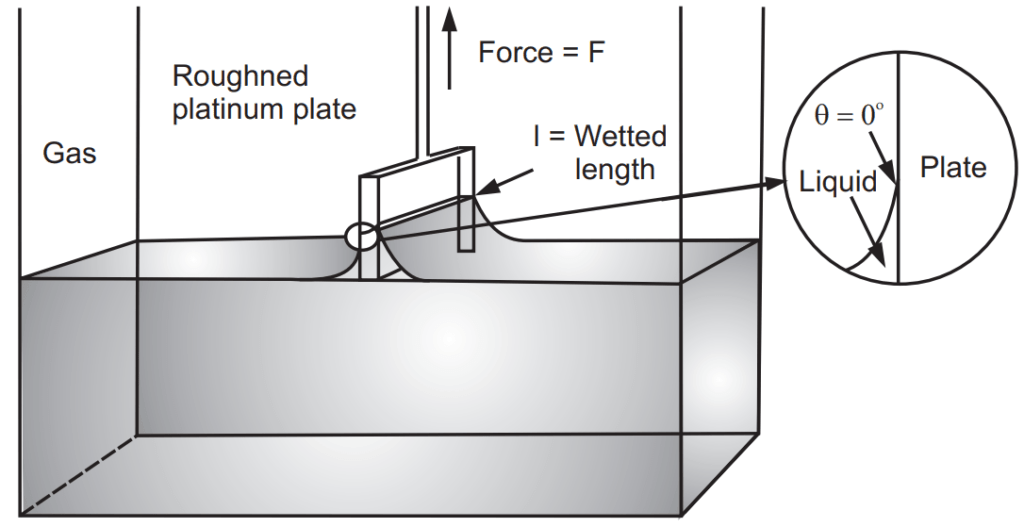
In the Wilhelmy plate method, the liquid is raised until the contact between the surface and the plate is observed. The maximum tension acts on the balance in this instance; this means that the sample does not need to be moved again during the measurement. Fig. 1.1 shows the illustrative diagram of the Wilhelmy plate. The following equation (below the equation) makes the surface tension calculation
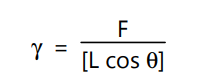
where γ is surface or interfacial tension, F is the force acting on the balance, L is the wetted length and θ is the contact angle. The plate is made of roughened platinum and is optimally wetted so that the contact angle is virtually a 0°. This means the term cos θ has a value of approximately the measured force and the length of the plate needs to be taken into consideration. Correction calculations are not necessary with the plate method.
Advantages of Wilhelmy Plate Method
- No correction is required for measured values obtained by this method.
- The densities of the liquids don’t have to be known.
- In an interfacial tension measurement, the surface is only touched and not pressed into or pulled out of the other phase; this avoids the phases becoming mixed.
- This method is used for static measurement i.e. the plate does not move after the surface or interface has been detached. The surface or interface renewal and ultimately measurement failure is avoided.
Disadvantages
- The wetted length surface is small, so small force is required leading to variation in results.
- Not suitable for cationic surfactants as platinum has poor wetting properties.
Make sure you also check our other amazing Article on : Ring Detachment Method