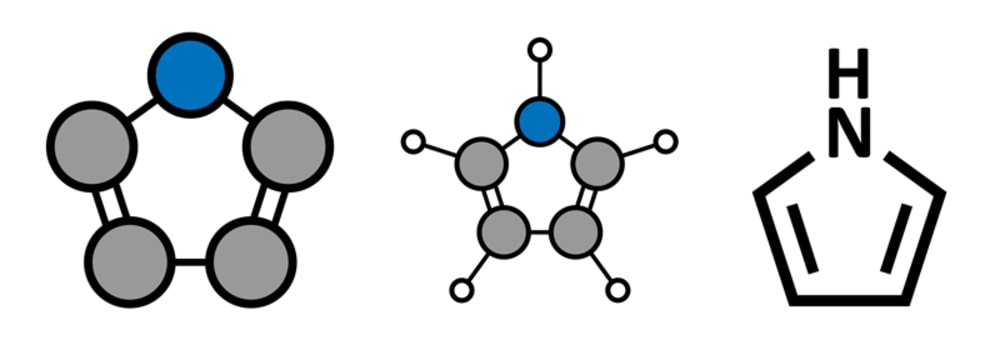
Pyrrole Synthesis: Pyrrole was first isolated from coal tar in 1834. It is an aromatic heterocycle having a weak aniline-like odor. It is a colorless volatile liquid that like aniline darkens by autoxidation. It has a boiling point of 129 to 131°C.
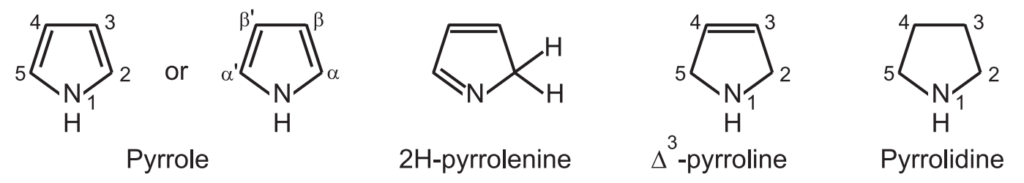
Pyrrole has three pairs of delocalized π electrons. Two of the pairs are shown as the bonds and the third pair is shown as a lone pair of nonbonding electrons on the nitrogen atom.
Pyrrole Synthesis
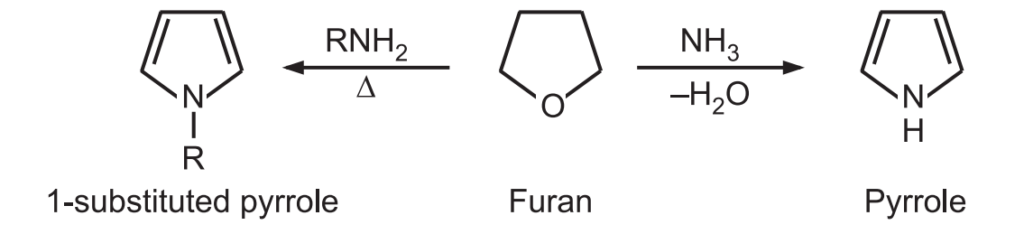
(1) Pyrrole is prepared industrially from furan by passing it over ammonia and steam and heated at 400°C in the presence of solid acid catalysts like SiO2 and Al2O3.
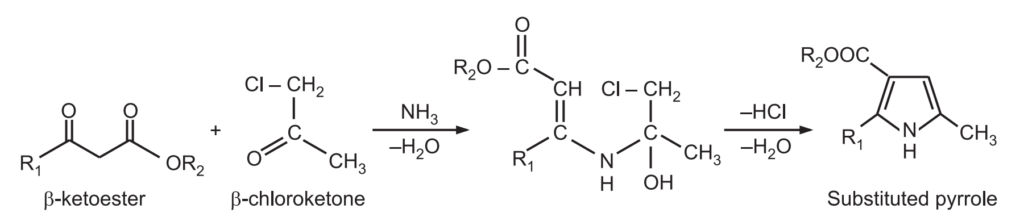
(2) Hantzsch Pyrrole synthesis: When α-halo ketone or aldehyde is reacted with a β-ketoester or β-chloromethane and a base like ammonia/primary amine, it gives pyrrole. The base functions both as a reactant and as a catalyst.
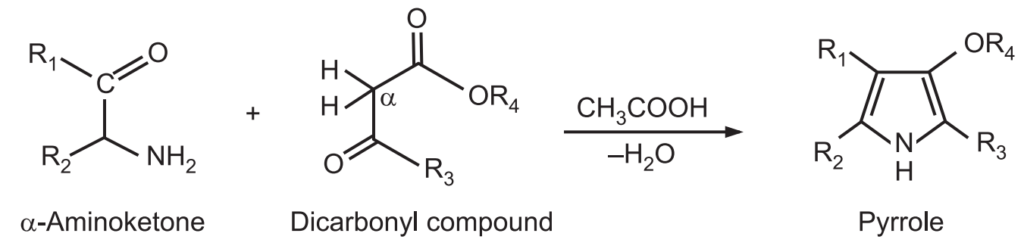
(3) Knorr Synthesis: In this widely used method, α – amino ketone is condensed with another dicarbonyl compound containing an electron-withdrawing group α to a carbonyl group (i.e., activated methylene group) in the presence of acetic acid.
(4) Paal-Knorr Pyrrole Synthesis: It is a condensation reaction between a 1,4-dicarbonyl compound with ammonia or a primary amine to form a substituted pyrrole. Pyrrole itself is formed from succinaldehyde and ammonia.
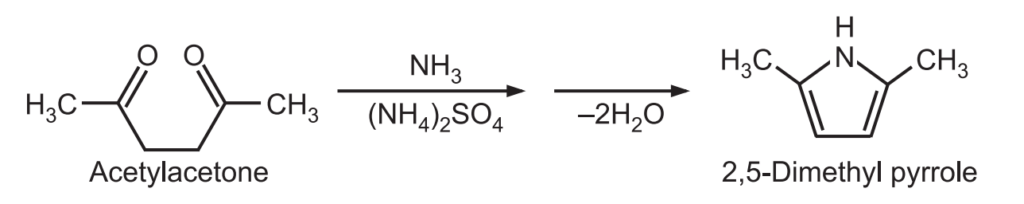
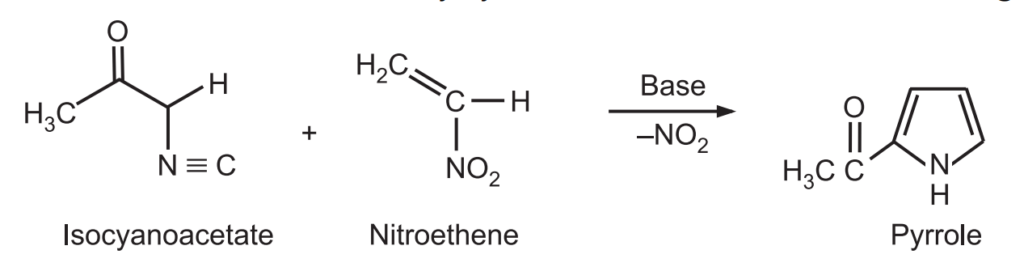
(5) Barton-Zard Synthesis: Pyrrole is obtained when an isocyanoacetate reacts with a nitroalkene in a 1, 4 – addition, followed by cyclization and elimination of the nitro group.
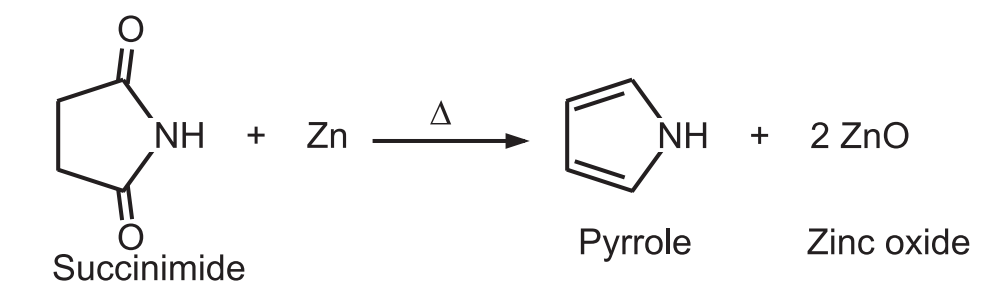
(6) From the distillation of succinimide: Pyrrole is obtained by the distillation of succinimide with zinc dust.
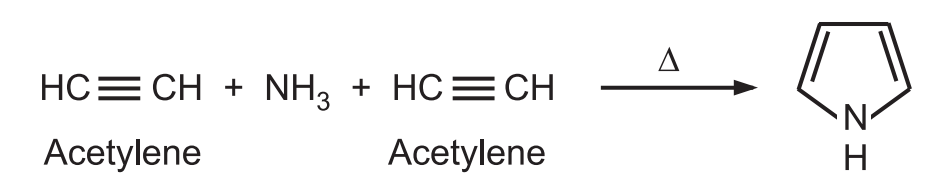
(7) From acetylene and ammonia: Pyrrole is obtained by passing acetylene and ammonia through a red hot tube.
Make sure you also check our other amazing Article on : Classification of Heterocyclic Compounds