Laboratory studies form an important part of assessing the quality of products. For test results to be accepted at national and international levels, they must be proven to be reliable. This is possible only when the systems in those laboratories meet certain quality requirements. The process of certifying this is called accreditation.
Table of Contents
Definition of Accreditation
Accreditation of laboratories is a process through which an authorized, independent agency examines and certifies the competence and quality systems of a laboratory based on particular predefined standards.
Accreditation provides formal recognition of the technical competence of a laboratory for particular measurements or tests based on results from a third-party assessment.
Advantages of Accreditation:
- Higher level of confidence in calibration/testing reports that the laboratory issues.
- Increased business with greater customer confidence in testing reports.
- Improved control over laboratory operations to maintain a sound quality management system.
- Guarantee of reliable test results, reducing the need for re-testing, resulting in savings.
- Improved visibility in the market as a good quality service provider.
Accreditation versus ISO 9000 Certification:
Accreditation is recognition of the technical competence of a test service provider or Conformity Assessment Body (CAB). Thus, it is a step beyond the system certification provided by ISO 9000. ISO certification will evaluate the CAB’s system for quality management; however, it will not give any insight into its ability to provide accurate and reliable test data or its technical competence in testing. By assessing the CAB for compliance with internationally accepted criteria, accreditation provides a greater level of information than mere ISO certification.
In most countries, accreditation for laboratories is mandatory; in India, however, it is a voluntary exercise.
India’s Accreditation Body – NABL:
In India, this body is named the National Accreditation Board for Testing and Calibration Laboratories – NABL. This body is a signatory of the Mutual Recognition Agreement with the regional body called Asia Pacific Laboratory Accreditation Cooperation and the apex body called International Laboratory Accreditation Cooperation. By this, NABL accredited laboratories can also achieve international recognition.
NABL is an autonomous body that is under the Department of Science and Technology, Government of India. It was first established to provide accreditation to laboratories involved with testing and calibration. Later, its scope was extended to accreditation for clinical laboratories, too. This is the only accreditation body authorized by the Government of India for the testing and calibration laboratories.
Standards:
Internationally, the acceptable standard for laboratories is ISO 15189. NABL provides accreditation in keeping with ISO/IEC 17025: 2005 “General Requirements for the Competence of Testing and Calibration Laboratories” and ISO 15189: 2012 ‘Medical laboratories — Requirements for quality and competence’.
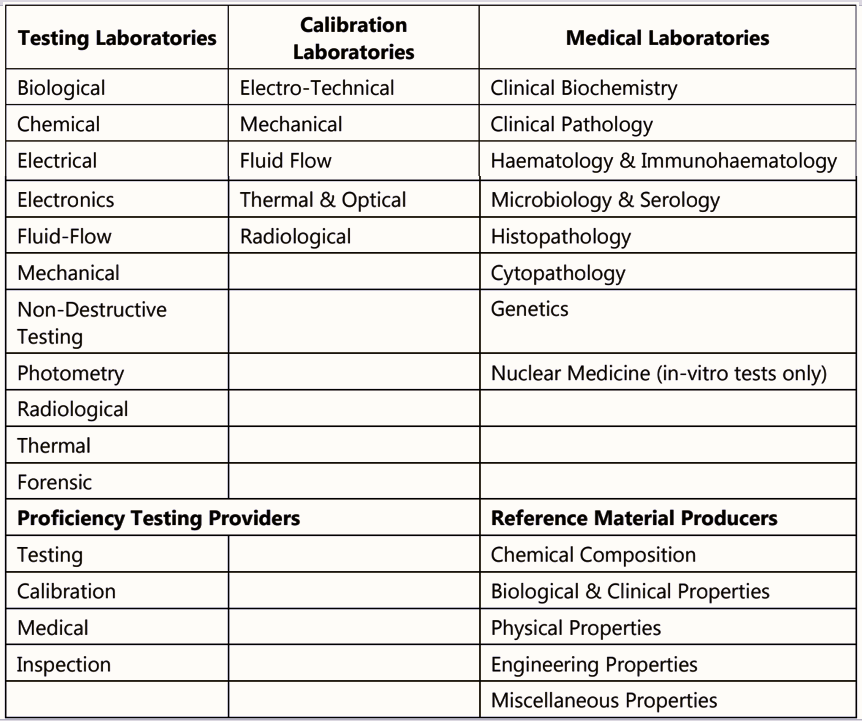
Source: NABL India website
How often accreditation must be done?
For it to be effective in maintaining quality performance and reliability of results, there must be done periodically, at regular intervals. NABL accreditation has a validity period of 2 years. The CAB must apply for accreditation renewal a minimum of 6 months before the accreditation validity period expires.
Procedure for NABL Accreditation:
CABs must follow the following process to obtain accreditation:
Step 1: CAB applies in the prescribed form in triplicate and attaches two copies of their quality manual describing their management system as per ISO or IEC guidelines as applicable. CAB also pays the application fee prescribed by NABL.
Step 2: On receiving the application, quality manual, and fee, NABL Secretariat issues an acknowledgment number with a unique ID number. This must be used for any correspondence. NABL scrutinizes the application and may ask for any clarification or information as necessary.
Step 3: NABL appoints a lead assessor who organizes a pre-assessment visit to the CAB premises. During this visit, any non-conformity in quality system implementation is evaluated.
This visit helps to evaluate:
- How prepared CAB is for the assessment?
- How many assessors will be required?
- Which key locations must be visited?
Step 4: Lead assessor submits a report of the pre-assessment visit to the NABL Secretariat. A copy is sent to the CAB too.
Step 5: CAB takes corrective measures regarding the non-conformity pointed out in the pre-assessment report. CAB sends a report on this to NABL Secretariat.
Step 6: Based on the satisfactoriness of the CAB’s corrective actions, NABL sets up the assessment team after consulting with the CAB. Along with the lead assessor, this team will include experts in the fields in which accreditation is sought by the CAB. Sometimes, an observer may also be nominated.
Step 7: Assessment team visits the CABs site. It reviews its systems and verifies compliance with the relevant certification standards. The team evaluates the CABs’ technical competence and identifies non-conformities that may be present.
Step 8: Assessment team prepares an assessment report, and based on the findings, recommends if accreditation may be granted or not. This report is sent to the NABL Secretariat. A copy of the report is given to the CAB at the end of the inspection.
Step 9: NABL examines the assessment report and initiates any follow-up action for non-conformities. It monitors the corrective actions taken by the CAB.
Step 10: Once all non-conformities have been addressed, the CAB is granted accreditation by the Chairman, NABL. The accreditation certificate will have the NABL hologram, unique number, discipline, and scope of accreditation along with the validity date.
If CAB does not agree with the decisions of the NABL, it may appeal to the Director, NABL with necessary information.
NABL will survey the accredited CAB annually to ensure they continue to comply with certification requirements.
Preparing for Accreditation:
When a CAB wishes to apply for accreditation, they must prepare in advance for the process. Here are some important steps to be performed.
- Obtain NABL documents from the NABL Secretariat to get familiar with the assessment process and details required for the application.
- Ensure training of one person by NABL on Quality Management System and Internal Audit.
- Prepare a quality manual in keeping with the standards.
- For each test/investigation done in the laboratory, prepare a Standard Operating Procedure (SOP).
- Calibrate instruments and equipment used in testing; ensure correct environmental conditions are maintained in the laboratory.
- Train personnel on documentation aspects.
- Check the status of the current technical competence and quality system by comparing with NABL standards and address deficiencies encountered.
- Prepare Quality Manual and all other documents required by NABL.
- Include Internal Quality Control (IQC) in sample analysis.
- Take part in External Quality Assessment Schemes (EQAs).
- Evaluate compliance with IQC and EQAs; take corrective actions where necessary.
- Perform internal audit and management review to assess preparedness for NABL assessment.
- After everything is satisfactory, apply for accreditation to NABL with the prescribed fees.
Laboratory testing is a vital component of the health care system. Accreditation is an efficient tool to assess the quality of laboratory services and is therefore highly beneficial to healthcare providers. For it to be effective in maintaining quality performance and reliability of results, accreditation must be done periodically, at regular intervals.
Make sure you also check our other amazing Article on: International Organization for Standardization(ISO)
