Aim: To determine the density of zinc oxide by liquid displacement method.
Principle: The density is the weight per unit volume. For solids, it is determined psychometrically from the liquid displaced by the submerged solid. When a solid mass is immersed in a liquid in which it is insoluble, it will displace an equal volume of liquid. That is
the volume of solid mass = the volume of the displaced liquid.
Apparatus and Materials required: Pycnometer (specific gravity bottle), zinc oxide, water
Process:
- About 1g of material (zinc oxide) is filled into a dried and tared (pre-weighed) specific gravity bottle and weighed.
- The specific gravity bottle containing zinc oxide is filled with water and weighed.
- After cleaning, the same specific gravity bottle is filled with water and weighed.
Observation:
Room Temperature: °C
Weight of empty dry specific. gravity bottle = a g
Weight bottle containing around I g of zinc oxide = c g
Weight of bottle containing zinc oxide and filled with water = d g
Weight of bottle fi lied with water = bg
Calculation:
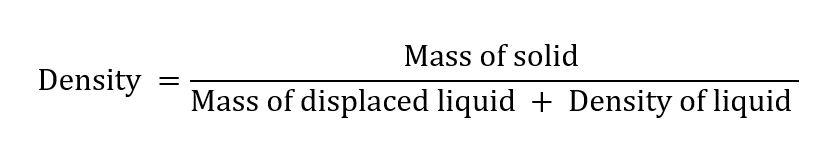
Mass of solid = (c – a) g
Mass of displaced liquid = mass of total liquid – a mass of the volume of the liquid present with the solid = (b – a) – (d – c)
The density of water at room temperature can be referred to from the table in the appendix.
Multiply the density value in g/ml by 103 to get the density in SI unit: kg/m3
Report: The density of zinc oxide isis kg/m3 at temperature __________ 0C.
(Density of few solids in g/ml:
Calcium oxide: 3.3
Zinc oxide: 5.6
Sodium chloride: 2.16
Kaolin: 2.2 to 2.5)
Make sure you also check our other amazing Article on: Determination of Distribution Coefficient Involving Association