Aim: To determine the distribution coefficient (partition coefficient) of benzoic acid between benzene and water.
Principle: If the solute exists in the same molecular state in both solvents, the simple concentration ratio of the solute in solvents represents the constant, distribution coefficient.
However, if, in one solvent, the solute exists as normal molecular species while in other it is associated or dissociated, then the simple concentration ratio is no longer constant.
If association occurs in one phase and n molecules of the solute combine together to form a complex molecule; then the distribution coefficient (partition coefficient) calculation of the nth root of concentration of the associated form is used.
Benzoic acid exists in water as a monomer and in benzene as a dimer (association). The concentration ratio between water and benzene can be written as
and similarly the ratio between benzene
Apparatus and Materials required: Stoppered glass bottles, benzoic acid, benzene, Nil 0 sodium hydroxide solution, phenolphthalein indicator, pipettes, and burettes.
Process:
- About 1, 2, and 3 g quantities of benzoic acid are transferred into three labeled stoppered glass bottles.
- Around 50 ml of benzene and around 50 ml of water are added to each bottle.
- After tightly stopping the bottles, they are vigorously shaken every 3 to 5 minutes for about one hour. The stoppers are removed to release the pressure.
- The bottles are then kept undisturbed to get the layers separated. The lower layer is aqueous and the upper layer is organic (benzene). (The separating funnels may be used instead of stoppered glass bottles).
- 10 ml of the benzene layer is pipetted out into a conical flask containing around 30 ml water. The solution is then titrated against N/I0 sodium hydroxide using phenolphthalein as an indicator. (During titration the contents should be shaken vigorously to ensure rapid and complete extraction of benzoic acid from benzene). Similarly, a 10 ml benzene layer of other bottles is also titrated.
- 10 ml of the aqueous layer is pipetted out into a conical flask and titrated against N/50 sodium hydroxide using phenolphthalein as an indicator.
Similarly, 10 ml of aqueous layer of other bottles are also titrated.
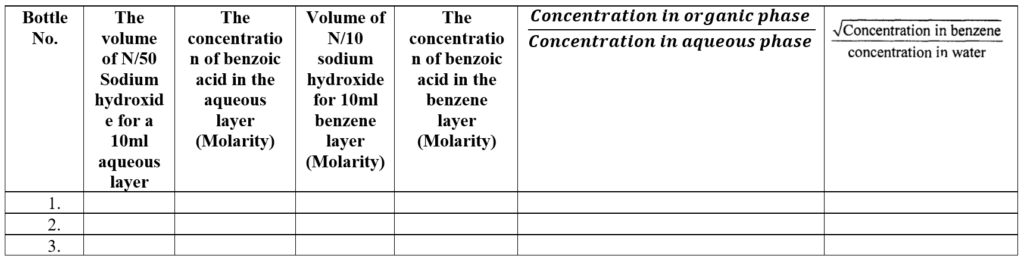
The normality and Molarity of benzoic acid are the same.
The concentration in Normality can be obtained from the relationship:
S1 x V1 = S2 x V2
where S1 and V1 are strength in Normality and volume in ml of sodium hydroxide; and S2 and V2 are strength in Normality and volume in ml of benzoic ·acid.
(1 Normal sodium hydroxide == I Normal benzoic acid)
Report: The distribution coefficient of benzoic acid between benzene and water is at °C.
Make sure you also check our other amazing Article on: Determination of Distribution Coefficient Without Association Dissociation