Differences Between Cosmetics And Cosmeceuticals
Introduction
- Raymond E. Reed (President of the Society of Cosmetic Chemists) in 1962, coined the term ‘’Cosmeceutics’’ he further defined cosmeceutics as a product that produces a useful and desired effect, gives desirable aesthetic properties, meets fixed chemical, physical, and medical standards, and is a scientifically designed product to apply to the human body externally.
- The definitions give rise to a product, that is of higher quality than cosmetics, but lower in standards than pharmaceuticals.
- Cosmeceutics is in fact a marriage between cosmetics and pharmaceutics, bringing therapeutic effects to mankind.
- Kligman AM, in the 1970s further augmented the term in a meeting of the Society of Cosmetic Chemists, he coined the idea of cosmetics being a hybrid of cosmetics and pharmaceuticals.
Origin of the Cosmeceuticals Concept
- The US Congress in 1938 officially defined cosmetics and drugs in specific terms, setting up formal criteria for classifying a product as either a drug or a cosmetic.
- No intermediate category was acceptable.
- The 1938 act came into being as a corrective reaction against the unregulated sale of innumerable patent medicines, some dangerous, which promised cures for all known human ailments.
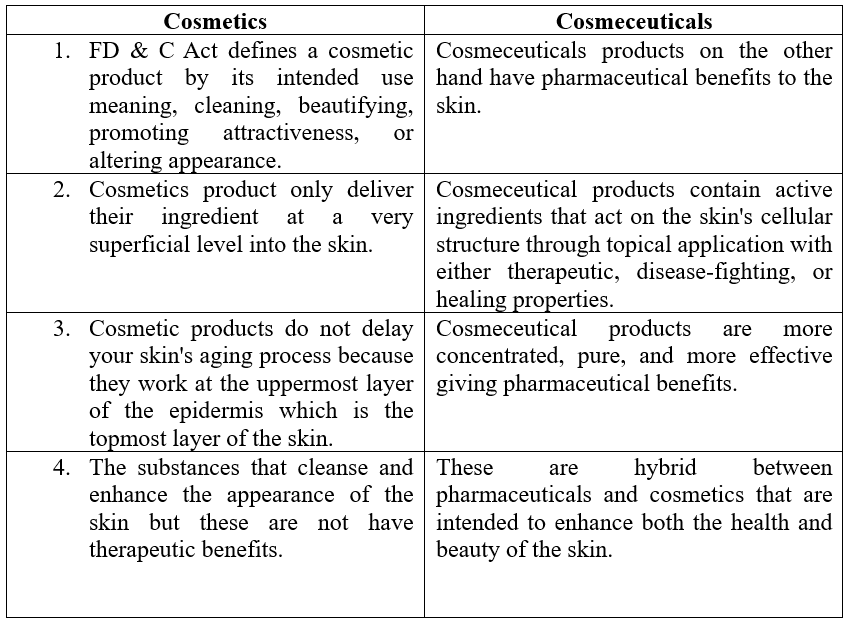
- The act defined a cosmetic as an “article intended for beautifying and promoting attractiveness”. In contrast, a drug is defined as a substance for use in the diagnosis, cure, treatment, or prevention of disease.
- Congress declared that the “intended” use would determine a product’s classification. Thus, if the intended use relates to the diagnosis and treatment of a disease, the substance is a drug, if its intended use is limited to promoting attractiveness, the substance is a cosmetic.
International Scenario of Cosmeceuticals
- Cosmeceutical is not a legal term nor is it acknowledged by the Food and Drug Administration (FDA).
- The Federal Food, Drug, and Cosmetic Act (FDC Act) categorizes products as cosmetics or drugs according to their intended use.
- The term ‘cosmeceutical’ enables us to classify more precisely a product with an activity that is intended to treat or prevent a (mild) skin (abnormality). In order to avoid any confusion with cosmetic definition, cosmeceuticals are regarded as a subclass within the domain of a cosmetic or drug.
- Cosmeceuticals can be regarded as a subclass of cosmetics in Europe and Japan, however in the United States, regarded as a subclass of drugs.
Cosmeceuticals characterized as
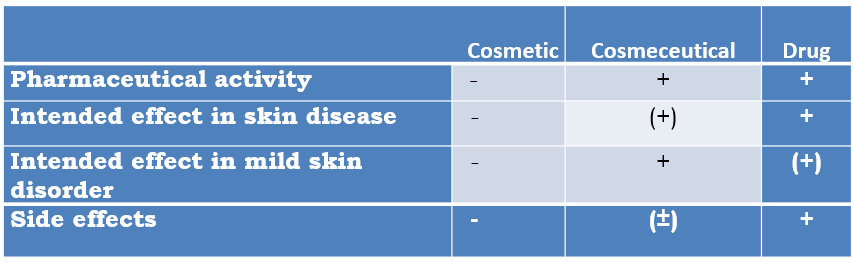
- The product has pharmaceutical activity and can be used on normal or near-normal skin.
- The product should have a defined benefit for minor skin disorders (cosmetic indication).
- As the skin disorder is mild the product should have a very low-risk profile.
Cosmeceuticals as a Subclass of Cosmetics (Europe and Japan) and as a Subclass of Drugs (U.S.)
Categorization of Cosmeceuticals in international markets
JAPAN: Japan accommodates cosmeceuticals by calling them “quasi-drugs”. These products exert mild actions on the human body.
- The ingredients included in quasi-drug must be pre-approved before being marketed in Japan.
- All products claiming to be cosmeceuticals are considered quasi-drugs and require pre-approval before selling in the market.
NEW ZEALAND: New Zealand law provides a third category called “related products”.
- Related products are those having therapeutic use as a purpose secondary to the main use.
- Related products labeled with an appropriate designation and trade name, the active ingredients to be disclosed quantitatively, the product’s true nature, expiry date and batch number, a dose and its frequency, directions for use, and name and address of the manufacturer.’
KOREA: The Korea Food and Drug Administration (KFDA) has classified cosmeceuticals or borderline products as “functional cosmetics” such as skin whitening, anti-aging, and sun care products.
- Functional cosmetics prevent melanin pigmentation, and spots, promote whitening of the skin, remove skin wrinkles, and block or diffuse UV rays to protect the skin.
- The KFDA is responsible for evaluating and improving the safety of functional cosmetics.
THAILAND: Under the current cosmetic regulation, cosmetics are classified as “controlled cosmetics” according to the ingredients used.
- The use of controlled ingredients as part of cosmetic products will require the notification of the products to the FDA before being marketed in Thailand.
AUSTRALIA: In Australia the categorization of goods is based on two factors viz., (a) Claims made about the product and (b) The composition of the product.
- Products that are at the borderline are classified as therapeutic goods. These goods must use only approved ingredients.
- The goods must be included in the Australian Register of Therapeutic Goods.
- Safety and efficacy and Good Manufacturing Practices (GMP) data must be submitted to regulatory authority.
CANADA: In Canada, cosmeceuticals are called as “dermocosmetics”.
- Canadian health authorities do not officially recognize cosmeceuticals as an independent cosmetics category.
INDIA: In India, the borderline products are cosmetic in one market and the same product is an OTC drug, non-prescription drug, or medicinal product in another market.
- After analyzing the above legislation, regulations, guidelines, and accepted practices for borderline products or cosmeceuticals in major countries, an attempt was made to prepare the much-needed regulatory framework for cosmeceuticals in India.
Make sure you also check our other amazing Article on: Hair Growth Cycle
