Definition of Gastro retentive drug delivery systems
These are the special class of novel drug delivery systems designed to prolong gastric residence time (Stomach) by targeting site-specific drug release for prolonged periods of time in the upper gastrointestinal tract (GIT) for local or systemic effects.
Table of Contents
Approaches of GRDDS
Floating drug delivery systems (FDDS)
- Effervescent systems
- Noneffervescent systems
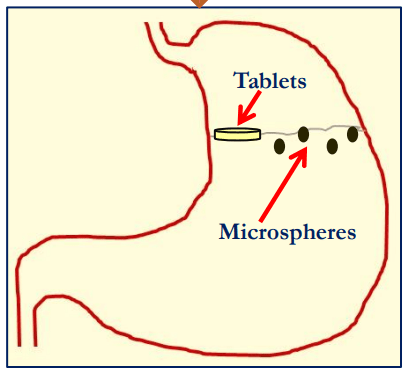
Drug delivery systems viz., Tablets or Microspheres Float immediately upon contact with gastric fluids.
Definition of Floating drug delivery systems (FDDS)
FDDS remain buoyant when the bulk density is less than the gastric fluid i.e., systems with a specific gravity lower than gastric contents viz., 1.004 – 1.01 gm/cm3
- The inherent low density can be provided by the entrapment of Air (e.g. hollow chambers), Low-density polymers, Volatiles liquids Gas generating agents, or Ion exchange resins.
- Minimal gastric content is needed to allow the proper achievement of the buoyancy retention principle.
- A minimal level of floating force (F) is required to keep the dosage form reliably buoyant on the surface of the meal.
- Buoyant provides prolonged gastric residence time and releases the drug slowly at a desired rate.
Effervescent systems
- Gas generating systems
- Gas or Liquid filled systems
Gas generating systems
Systems contain optimum concentrations of Gas generating agents usually CO2 generating agents viz., Sodium bicarbonate, Citric acid, or Tartaric acid along with novel swellable polymers viz., Methocel, Ethocel, Xanthan gum, Chitosan to achieve floatability.
After oral administration of these systems in the Stomach, CO2 is liberated which reduces the density of the system by the influence of novel polymers and making it float on the Gastric fluid.
Types of Gas generating systems
- Intragastric single-layered floating tablets
- Intragastric bi-layered floating tablets
- Multiple unit floating tablets
1. Intragastric single-layered floating tablets
- Formulated by intimately mixing the CO2-generating components and drug candidates within a tablet matrix.
- These have a bulk density lower than the gastric content and therefore achieve buoyancy in the stomach for a prolonged period of time.
- The drug is released from the matrix tablet in a sustained manner at a desired rate.
- After completion of the drug release, the residual system is to be expelled from the stomach.
2. Intragastric bi-layered floating tablets
Intragastric bi-layered floating tablets contain the gas-generating agents in the immediate release layer, the second layer. sustained release layer, which controls the drug release.
3. Multiple-unit floating tablets
- The system consists of sustained-release tablets coated or surrounded by double layers.
- The outer layer was a swellable membrane layer with tartaric acid.
- The effervescent layer was divided into two sub-layers to avoid direct contact between these two gas-generating agents viz., Sodium bicarbonate in the inner sub-layer, while tartaric acid in the outer layer.
- When the system was immersed in simulated systems it sank at once in the solution and formed swollen tablets, which float once density lowers. ü This lower density is due to the generation and entrapment of CO2 within this system.
Systems containing Volatile liquid systems/Inflatable systems
- This system is comprised of a hollow deformable unit, which is internally divided into two chambers separated by an impermeable, pressure-responsive movable bladder.
- The first chamber contains a drug reservoir, while the second chamber contains a volatile liquid, such as cyclopentane or ether.
- These volatile liquids vaporize at physiological temperatures to produce a gas, enabling the drug reservoir to float.
- To enable the unit to exit from the stomach, the device contained a bioerodible plug that allowed the vapor to escape
- These systems are incorporated in an Inflatable chamber, which contains a volatile liquid viz., ether, cyclopentane, and a Drug reservoir with bioerodible polymers.
- These liquids gasify at body temperature to cause inflammation of the chamber in the stomach.
- The polymers gradually dissolve causing the inflatable chamber to release gas enabling the systems to float.
- The chamber collapses after a predetermined time to permit the spontaneous ejection from the stomach.
Gas-filled floating delivery systems
- Gas-filled floating delivery systems include the incorporation of a gas-filled floatation chamber, which may be vacuum or filled with air or a harmless gas into a microporous component that houses a drug reservoir.
- Apertures or openings are present along the top and bottom walls through which the gastric fluid enters to dissolve the drug, while the other two walls in contact with the fluid are sealed so that the undissolved drug remains therein.
Non Effervescent systems
- Non-effervescent FDDS are normally prepared from gel-forming or highly swellable cellulose-type hydrocolloids, polysaccharides, or matrix-forming polymers like polyacrylate, polycarbonate, polystyrene, polymethacrylate, carpool, HPMC, sodium alginate, chitosan, etc.
- These systems can be further divided into the following sub-types
- Intragastric osmotically controlled floating delivery systems
- Hydrodynamically balanced systems HBSs
- Micro balloons (Hollow microspheres)
- Alginate beads
1. Intragastric osmotically controlled floating delivery systems
- These systems are enclosed in a bioerodible polymer plug that erodes after a predicted time period and tends to float in the fluid.
- The osmotic pressure floating systems basically consist of two compartments viz., a Drug reservoir compartment and an osmotically active compartment.
- The drug reservoir compartment is enclosed by a pressure-responsive collapsible bag, which is impermeable to vapor and liquid and drug delivery orifice.
- The osmotically active compartment contains osmotically active salts and is enclosed within a semipermeable housing.
- In the stomach, the water in the gastric fluid is continuously absorbed through the semipermeable membrane into the osmotically active compartment to dissolve the osmotically component.
- The osmotic pressure is then produced, which acts on the collapsible bag and in turn forces the drug reservoir compartment to reduce its volume and activate the release of drug candidates in the form of solution through the delivery orifice.
2. Hydrodynamically balanced systems HBSs
- HBS is a novel approach to increase the gastric residence time of drugs in the stomach.
- This system is designed for site-specific oral drugs with lower bulk density than gastric fluid so as to buoyant the dosage forms in the stomach to increase the residence time of the drug.
- These are single-unit dosage forms, containing one or more gel-forming hydrophilic polymers viz., HPMC, HEC, HPC, Na CMC, Polyacrylates, Polystyrene, Agar, Carrageenans or Alginic acid.
- When the HBSs containing drughydrocolloid mixture comes in contact with gastric fluid, the dissolves the polymers and the mixture swells to form a gelatinous barrier.
- This imparts buoyancy in gastric juice for a long period due to its continuous erosion of the surface, which allows water penetration to the inner layers maintaining surface hydration and buoyancy to the dosage form.
- Effective drug deliveries depend on the balance of drug loading and the effect of the polymer on its release profile.
3. Micro balloons (Hollow Microspheres)
- Micro balloons (hollow microspheres) novel FDDS loaded with drugs in polymeric shells prepared by simple solvent evaporation or solvent diffusion/ evaporation method to create a hollow inner core, which prolongs the GRT of the dosage form
- Polymers used are Polycarbonate, Cellulose acetate, Calcium alginate, Eudragit S, Agar low methoxylated pectin, etc.
- Buoyancy and drug release from dosage form are dependent on the quantity of polymers, the plasticizer polymer ratio, and the solvent used for formulation.
- The micro balloons floated continuously over the surface of an acidic dissolution media containing surfactant for >12 hrs.
- Microballoons or hollow microspheres are considered to be one of the most promising buoyant systems because they combine the advantages of single-unit and multiple-unit systems and good floating.
4. Alginate beads
- They were made by using Ca2+ and low methoxylated pectin (anionic polysaccharide), or Ca2+ low methoxylated pectin and sodium alginate.
- In this approach, generally sodium alginate solution is dropped into the aqueous solution of calcium chloride and causes the precipitation of calcium alginate.
- In another method, multiple-unit floating alginate beads have been developed by from freeze-dried calcium alginate using sodium alginate as the polymer and calcium chloride as a cross-linking agent.
- These beads are separated and dried by air convection and freeze drying, leading to the formulation of a porous system, which can maintain a floating force for over 12 hours.
- These beads improve GRT by more than 5.5 hrs.
High-Density Sinking Systems
- Sedimentation has been employed as a retention mechanism for high-density systems that are small enough to be retained in the folds of the stomach body near the pyloric region, which is part of the organ with the lowest position in an upright posture.
- This approach involves the formulation of dosage forms with a density that must exceed the density of normal stomach content (~ 1.004 gm/cm 3).
- These formulations are prepared by coating the drug on a heavy core or mixed with inert materials such as Iron powder, Barium sulphate, Zinc oxide Titanium oxide, etc.
- The materials increase density by up to 1.5- 2.4 gm/cm3 and a density close to 2.5 gm/cm3 seems necessary for significant prolongation of gastric residence time.
- The effectiveness of this system in human beings is limited.
Expandable, unfoldable, and swellable Systems
- Gastro retentivity of a pharmaceutical dosage form can be enhanced by increasing its size above the diameter of the pylorus.
- If the dosage form can attain a larger size than pylorus, the gastro retentivity of that dosage form will be possible for a long time.
- This large size should be achieved fairly quickly, otherwise dosage form will be emptied through the pylorus.
- Configurations required to develop an expandable system to prolong GRT are
- Small configuration for oral intake
- Expanded gastroretentive form
- Withstand peristalsis and mechanical contractility of the stomach
Unfoldable systems
- Different geometric forms like a tetrahedron, ring, or planar membrane (4-lobed, disc, or 4- 4-limbed cross form) of bioerodible polymer loaded with drugs compressed within a capsule viz., folded into large hard gelatin capsules (00 or 000 sizes)
- The concept is to expand or extend in the stomach. GRD of drug, based on unfolding polymer membranes that combine extended dimensions (5 cm x 2.5 cm).
- Delivery systems reached its unfolded form within 15 min
- These systems with extended size but with a lack of high rigidity are unable to retain in the stomach, which may cause brief obstruction and gastropathy.
- Therefore, the rigidity of these systems is also a crucial parameter in designing such a gastroretentive delivery.
Swellable systems
- Swellable systems are retained in the GIT due to their mechanical properties.
- The swelling of the dosage form is usually resulted from osmotic absorption of water and the dosage form is small enough to be swallowed by the gastric fluid.
- In general, these size-increasing drug delivery systems potentially present the hazard of permanent retention in the stomach and could lead to life-threatening effects upon multiple administrations.
- They are not cost-effective and the major advantage of these size-increasing systems is the independence of their performances on the filling state of the stomach.
Bioadhesive or Mucoadhesive systems
These systems are fabricated with suitable Bioadhesive polymers viz., HPMC, sodium CMC, chitosan, sodium alginate, sucralfate, tragacanth, dextrin, gliadin, lectin, etc.
These systems tend to adhere to the epithelial surface or mucosal surface by different mechanisms in the stomach, thus, increasing the GRT of the dosage forms.
Limitations
- Gastric mucoadhesion does not tend to be strong enough to impart to dosage forms the ability to resist the strong population forces of the stomach wall.
- The continuous production of mucous by the gastric mucosa to replace that is lost through peristaltic contractions and the dilution of the stomach content also seems to limit the potential of mucoadhesion as a gastroretentive force.
- The major challenge for bioadhesive drug delivery systems is the high turnover rate of the gastric mucus in the GIT and the resulting limited retention times.
- Very difficult to target specifically the gastric mucus with bioadhesive polymers.
Advantages
- Manipulate gastric retention time as well as gastric emptying time by buoyant of the system.
- Minimize the fluctuation of drug concentrations and effects.
- Improved patient compliance in achieving controlled drug release.
- Suitable for,
- Drugs that have narrow absorption windows in GIT viz., Furosemide, Riboflavin.
- Drugs that are locally active in the stomach viz., Misroprostol, Antacids.
- Drugs that are unstable in the intestinal or colonic environment viz., Captopril, Ranitidine HCl.
- Drugs that disturb normal colonic microbes viz., tetracycline, clarithromycin, and amoxicillin used for the eradication of Helicobacter pylori.
- Drugs that exhibit low solubility at high pH values viz., Diazepam, Chlordiazepoxide, Verapamil.
Make sure you also check our other amazing Article on: Functions of Pancreas