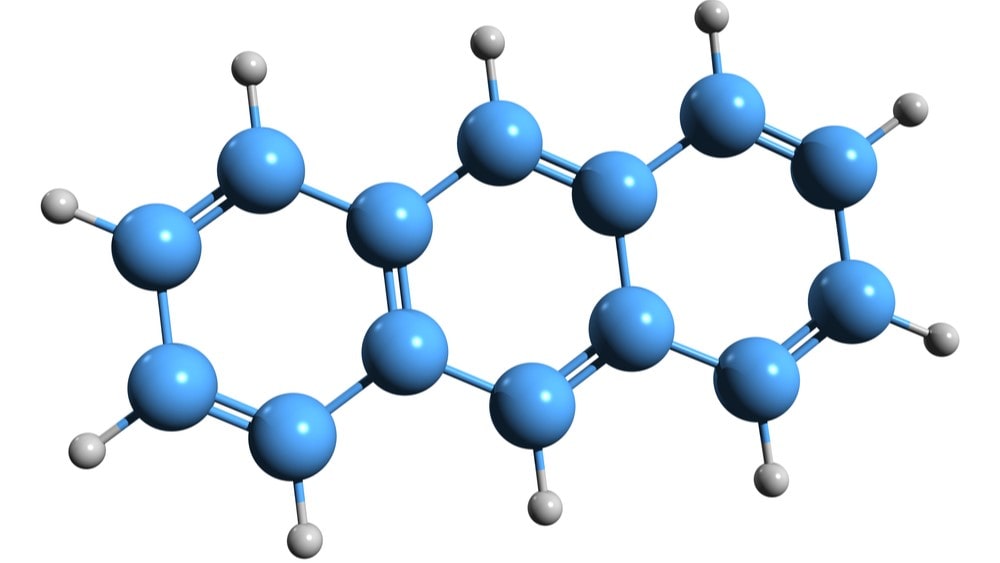
Due to its less aromaticity than benzene, anthracene of reaction give both electrophilic substitution and addition reactions and these reactions occur at position 9 or 10 since the carbocations produced due to the attack of an electrophile on position 9 or 10 are the most stable since aromatic sextet is preserved in two of the three rings.
Electrophilic substitution reactions:
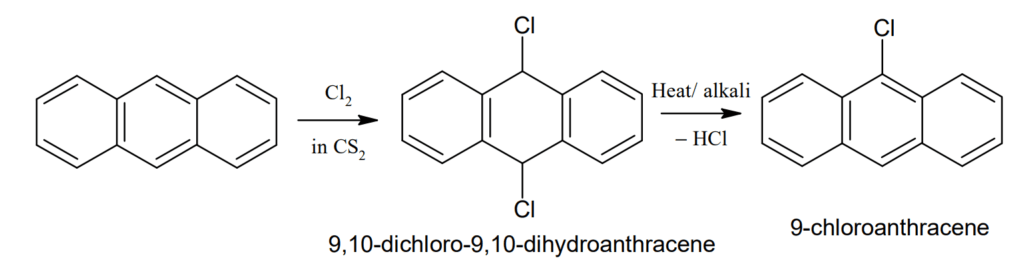
Halogenation
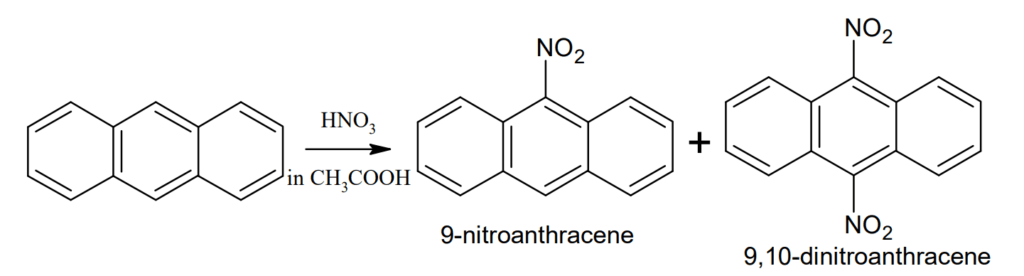
Nitration: Anthracene on nitration with aqueous nitric acid undergoes oxidation to form anthraquinone but with nitric acid in acetic acid at 15-20° C gives a mixture of 9-nitroanthracene and 9,10-dinitroanthracene
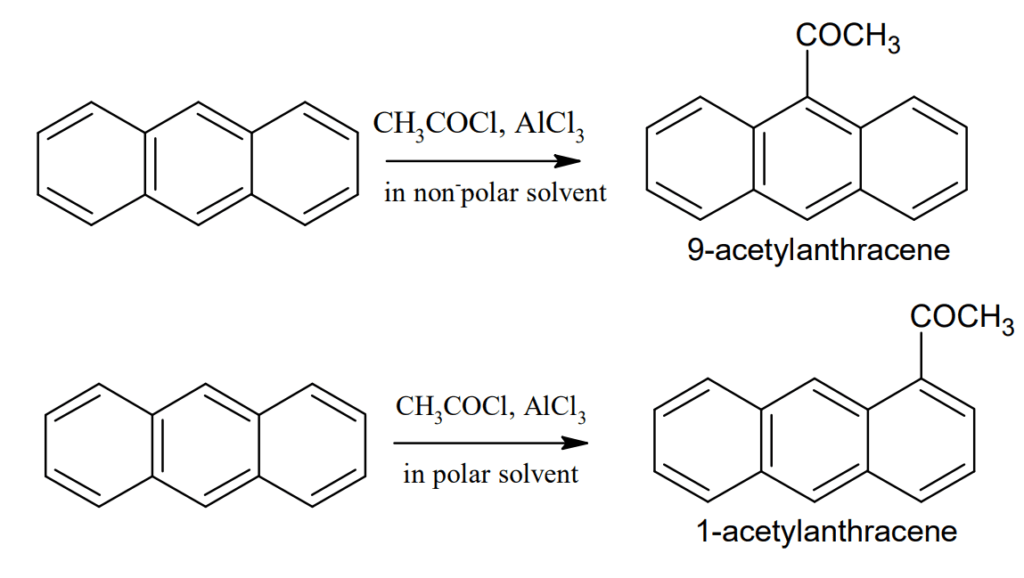
Friedel-Craft reaction: Friedel-Craft reaction occurs at 9- position in non-polar solvent but in presence of polar solvent it occurs at 1-position.
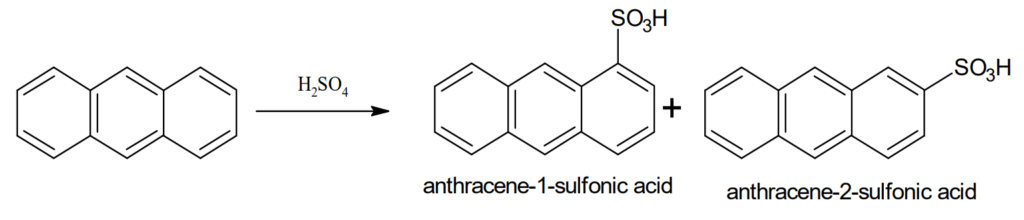
Sulphonation: Anthracene can be easily sulphonated to give a mixture of 1- and 2- sulphonic acids.
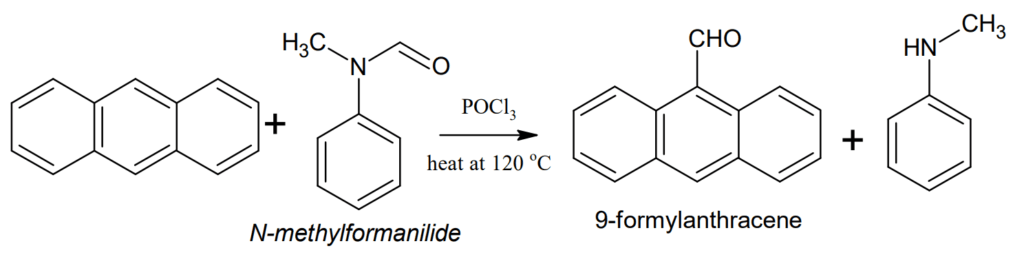
Formylation (Vilsmeier reaction): Anthracene can be formylated at 9-position when heated at 120 °C with N-methylformanilide in presence of POCl3.
Addition reaction:
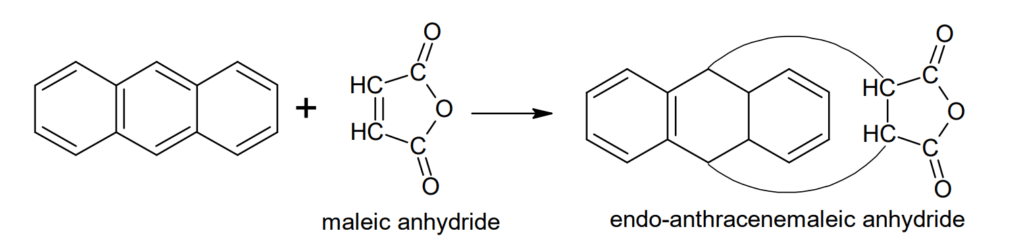
Diels-Alder reactions: Antharacene undergoes Diels-Alder reaction at 9- and 10- positions and forms endo-anthracene maleic anhydride.
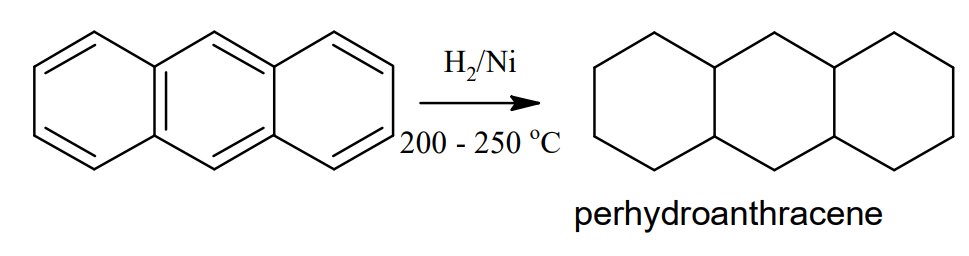
Reduction: When reduced with sodium and isopentanol, anthracene forms 9,10- dihydroanthracene. Catalytic reduction of anthracene using nickel at 200 – 250 °C makes tetra, hexa, octa, and finally perhydroanthracene (C14H24)
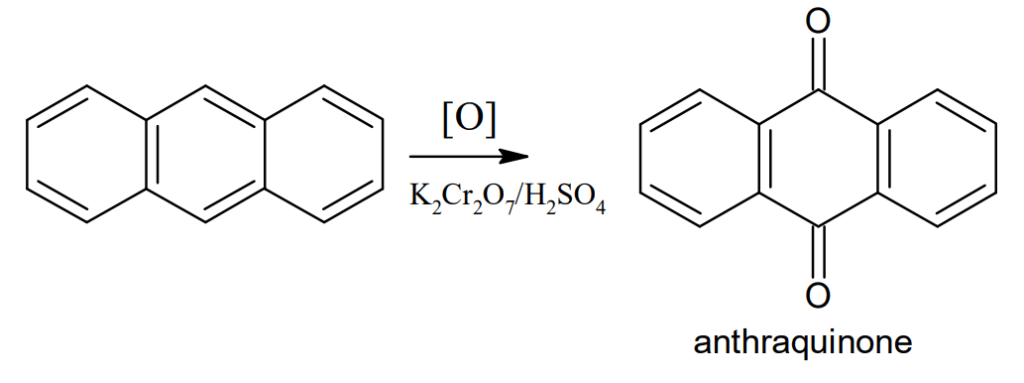
Oxidation: Anthracene on oxidation with potassium dichromate(K2Cr2O7) and sulphuric acid gives anthraquinone
Uses: Used as an insecticide for crops, wood preservative. Herbal drugs like senna, aloes, etc containing anthraquinones are used as laxatives/purgatives.
Make sure you also check our other amazing Article on : Resonance of Phenanthrene