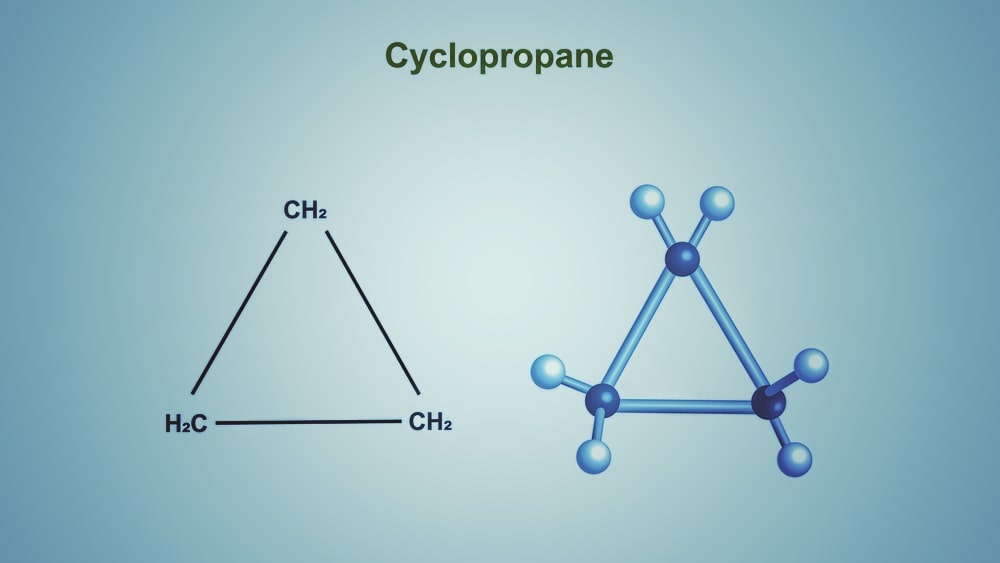
Reaction of Cyclopropane
(a) Addition reactions: These reactions lead to the opening of the ring to relieve the ring strain. In these reactions, the reagent attaches to two terminals of the resulting propane chain.
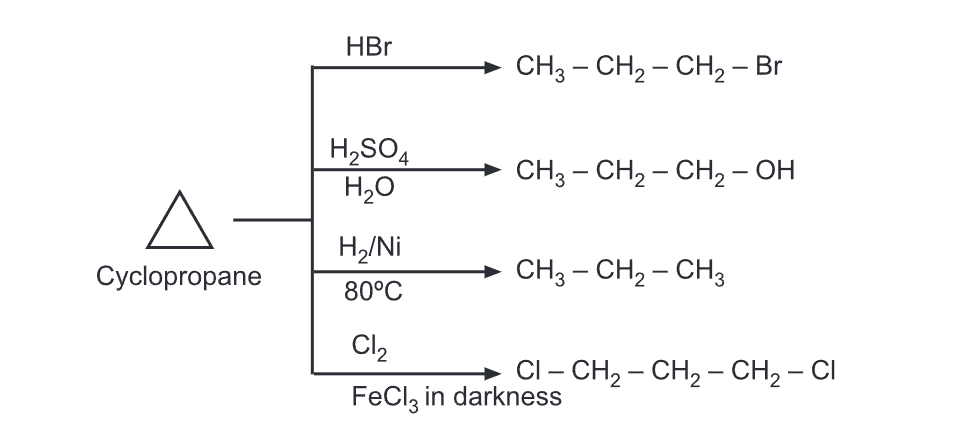
The electrophilic addition in substituted cyclopropanes generally follows Markovnikov’s rule wherein hydrogen adds carbon which already contains more hydrogens. Nucleophilic addition to cyclopropane is possible only when an electron-withdrawing group is present on the ring.
The addition of free radicals to cyclopropane is also reported. Thus bromine adds to a cyclopropane ring in presence of UV light via a free radical mechanism.
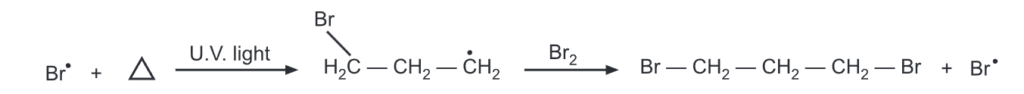
Reaction of Cyclobutane
Cyclopropane and cyclobutane are present in the gaseous state at room temperature while the remaining cycloalkanes exist in a liquid state. Due to angle strain, the C-C bonds in cyclopropane and cyclobutane are weak. Because of these weak bonds, cyclobutane undergoes reactions similar to that of cyclopropane.
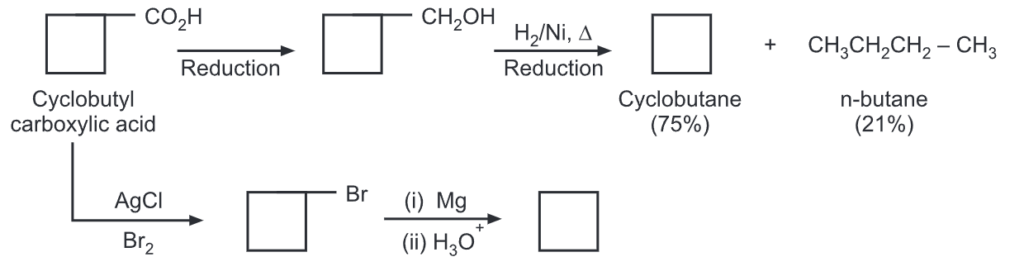
Cyclobutane (b.p. -150): It was first prepared by Willstatter (1907), using the Hofmann exhaustive methylation reaction.
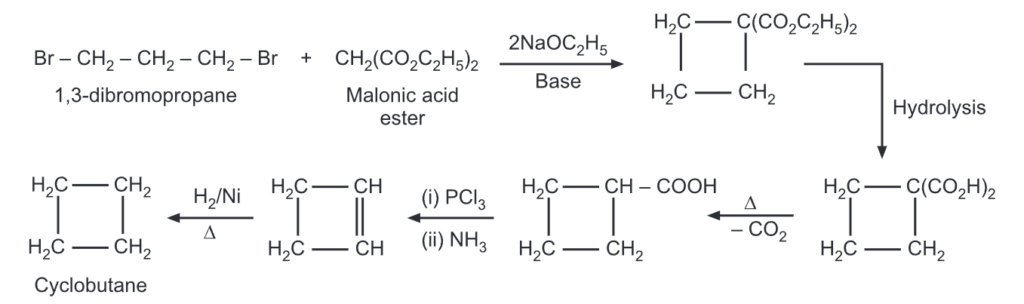
The yield of cyclobutane was obtained by ring closure of 1,4-halogen alkanes. In yet another method, cyclobutane carboxylic acid may be converted to cyclobutane by following routes.
Make sure you also check our other amazing Article on : Baeyer Strain Theory