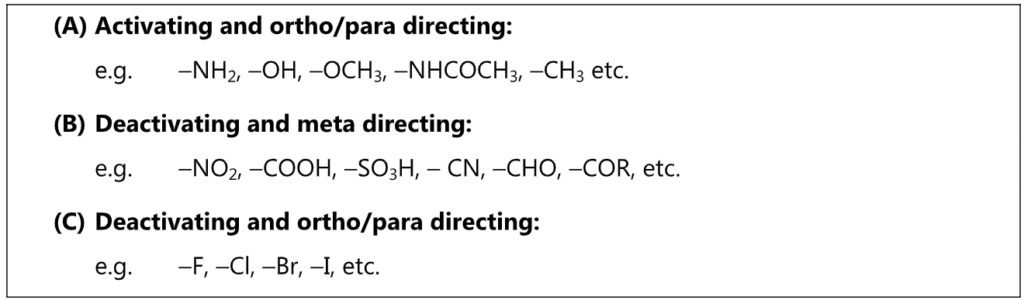
Theory of Orientation: Out of two important factors that govern the reactivity of aromatic rings towards an electrophile, the first involves the electron concentration in the aromatic ring before the attack. The second factor is the relative stabilities of the carbocation intermediates formed in electrophilic aromatic substitution. When an electrophile E attacks the mono-substituted benzene ring, the stability in terms of energy of the carbonium ion will govern the orientation of the next coming substituent.
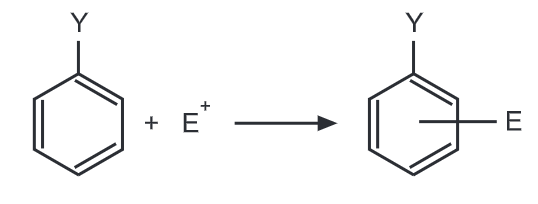
Regardless of its electron donating or electron withdrawing nature, Y exerts its influence more at ortho and para positions. Hence, ortho and para positions are more activated by an electron donating group while an electron-withdrawing group deactivates ortho and para positions more than it does meta.
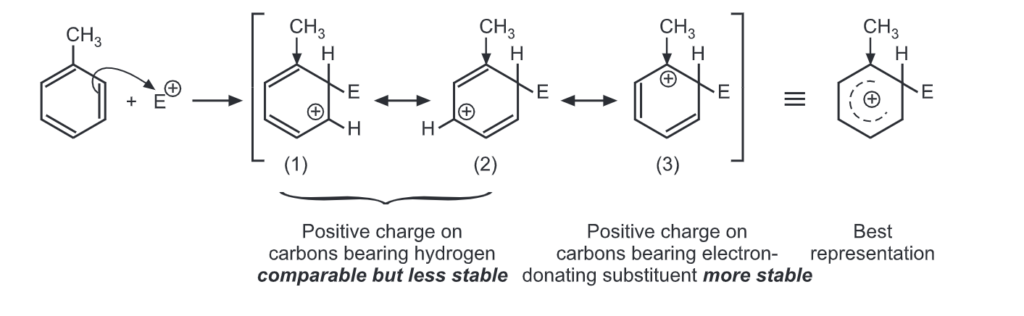
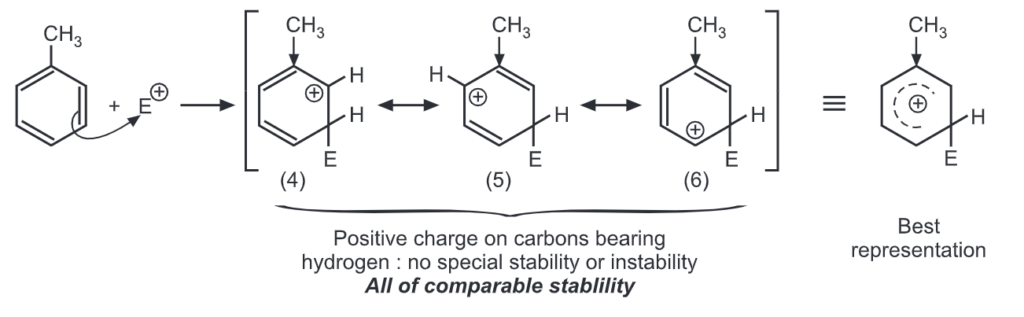
Let us consider the electrophilic substitution of phenol. The carbocations formed from ortho, meta and para attack by an electrophile, E+ are shown here. Theory of Orientation:
Ortho attack:
Meta attack:
Para attack:
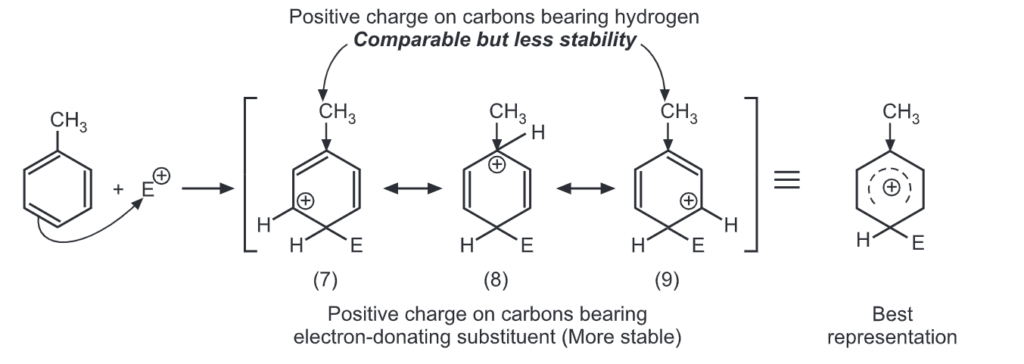
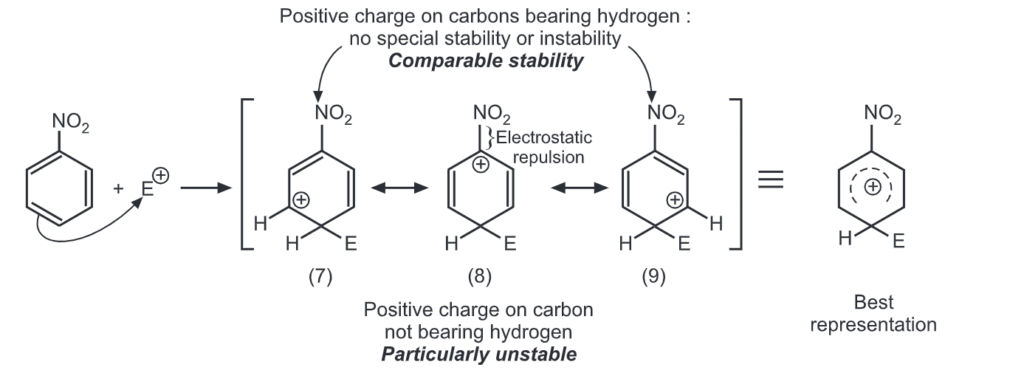
Out of nine carbocations generated, carbocations (3) and (8) are the most stable because of the electron-donating hydroxyl group on the carbon-bearing positive charge. Thus, we predict that ortho and para attacks are favoured over meta attacks in phenol. Similarly in the case of nitrobenzene, because of unstable carbocations (12) and (17), meta attack is favoured. Hence, the ortho and para substitution is slower than meta substitution.
Ortho attack:
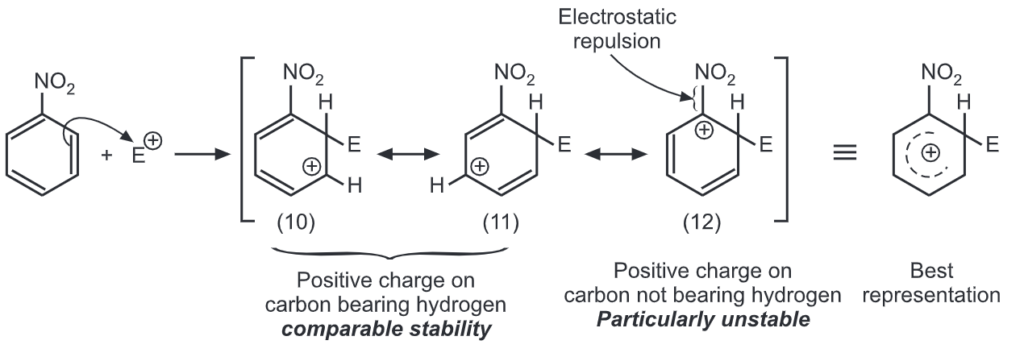
Meta attack:
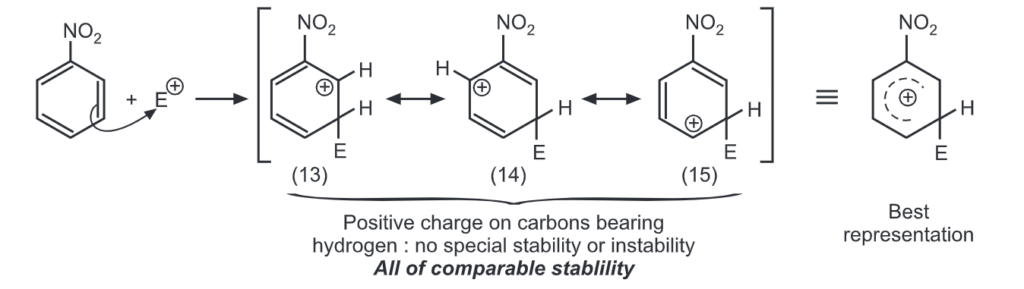
Para attack:
Halogens are unusual in their effect on electrophilic aromatic substitution. A halogen atom deactivates the ring toward further substitution compared with benzene yet it is ortho, para-directing. Because of the electron withdrawal through the inductive effect, halogen leads to a decreased affinity for the electrophilic attack on the ring and decreased stability of the carbocation that results. Hence, halobenzenes are less reactive than benzene. We would predict that halogens, such as chlorine, are meta directors. Chlorine contains three unshared pairs of nonbonding electrons and unshared electron pairs adjacent to an aromatic ring can donate electrons through the resonance effect. Due to the resonance effect, halogen tends to release electrons and stabilize intermediate carbocation.
Ortho attack:
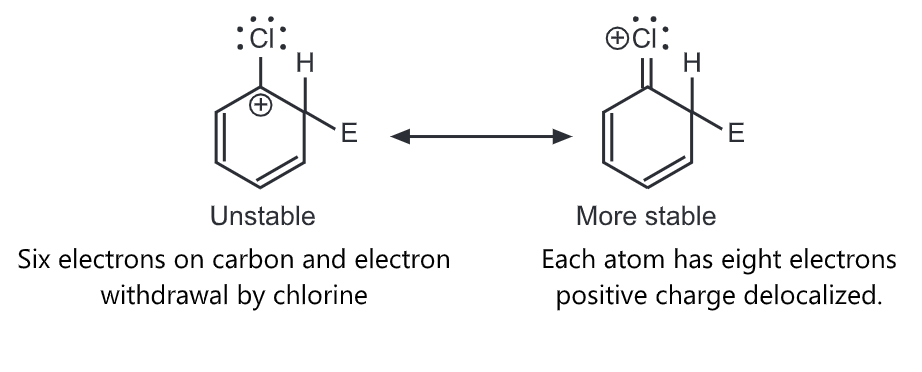
Para attack
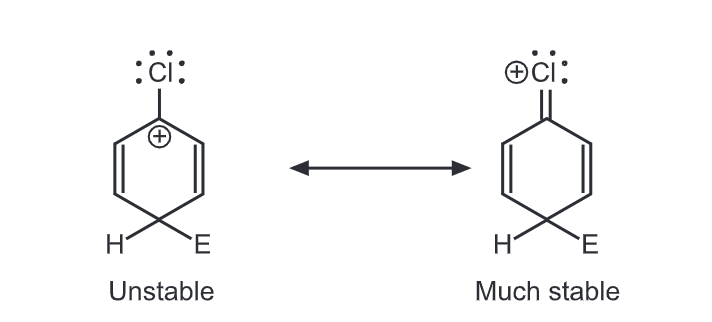
Thus, the inductive effect is largely responsible for decreasing reactivity while the resonance effect is largely responsible for governing orientation. In some substituents (-NH2, OH), the resonance effect completely dominates the inductive effect. With the halogens, these effects are more evenly balanced. Hence they show the unusual behaviour of being deactivating but ortho, para-directing.
The Aryl group, Ar, is an ortho, para directing and a weak activating group.
Make sure you also check our other amazing Article on : Huckel’s Rule