Dehumidification means decreasing humidity (removal of moisture) from the air.
Dehumidification is accomplished by bringing the moist air in contact with a cold surface (liquid or solid). Many large air conditioner plants incorporate automatic control of the humidity and temperature of the issuing air. Temperature control is effected with the aid of a thermocouple or resistance thermometer. Humidity is controlled using a thermocouple recording of the difference between the wet and dry-bulb temperatures.
Applications
Dehumidifiers are used in special heat transfer devices to liquefy vapour by removing their latent heat. Such systems are called condensers.
In the pharmaceutical industry, many operations are carried out at a stated humidity to get optimum results. In many parts of India (e.g., Bengal, Kerala etc.) the air is very humid and it becomes difficult to carry on operations with hygroscopic substances even in an air-conditioned room. Hence, dehumidifiers are installed for certain operations.
Much sophisticated electronic equipment is maintained in dehumidification conditions.
Mechanism of Dehumidification
The principle of dehumidification is illustrated in the Figures (Humidification). If the temperature of the surface is lower than the dew point of the gas, condensation takes place and the temperature of the gas falls.
The air in contact with the surface is cooled below its dew point. Subsequently, air from a distant place condenses, which requires time to cool. Therefore, effective mixing enhances dehumidification. Normally, the temperature and humidity are reduced simultaneously throughout the process. After dehumidification, the gas can be reheated to its original dry-bulb temperature.
The conditions at a particular point in a dehumidifier are shown in Figure 1. The x-axis indicates the perpendicular distance to the interface. The ordinate represents temperature and humidity. (Broken arrows represent the diffusion of vapour through the gas phase. Unbroken arrows represent the flow of heat (both latent and sensible) through the gas and liquid phase).
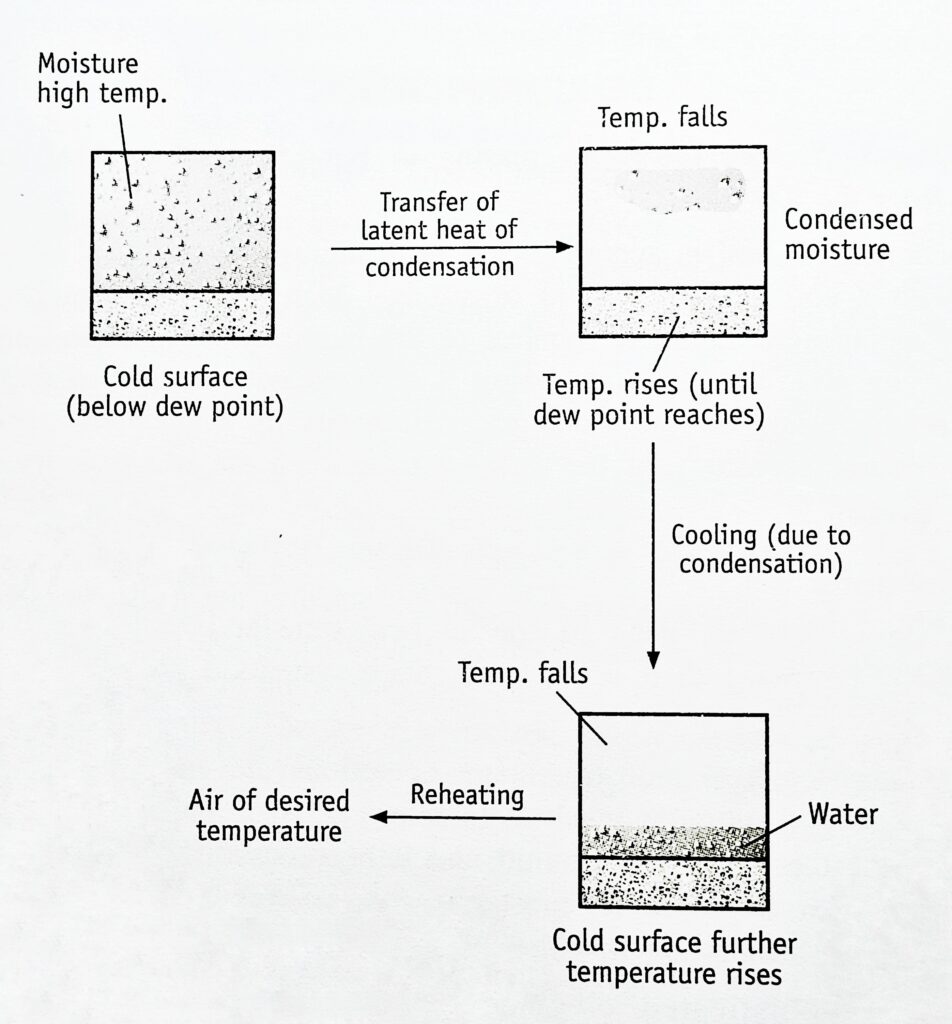
Temperature and humidity and reduced simultaneously.
In this case, the humidity of the air, Hy is greater than the humidity at the interface, Hi and, therefore, water vapour diffuses to the interface. Since vapour is condensed to water, latent heat is transferred to the water. So the temperature of the surface tends to rise and that of the air decreases. It would be expected that the air would cool at a constant humidity until the dew point is reached (Figure 2).
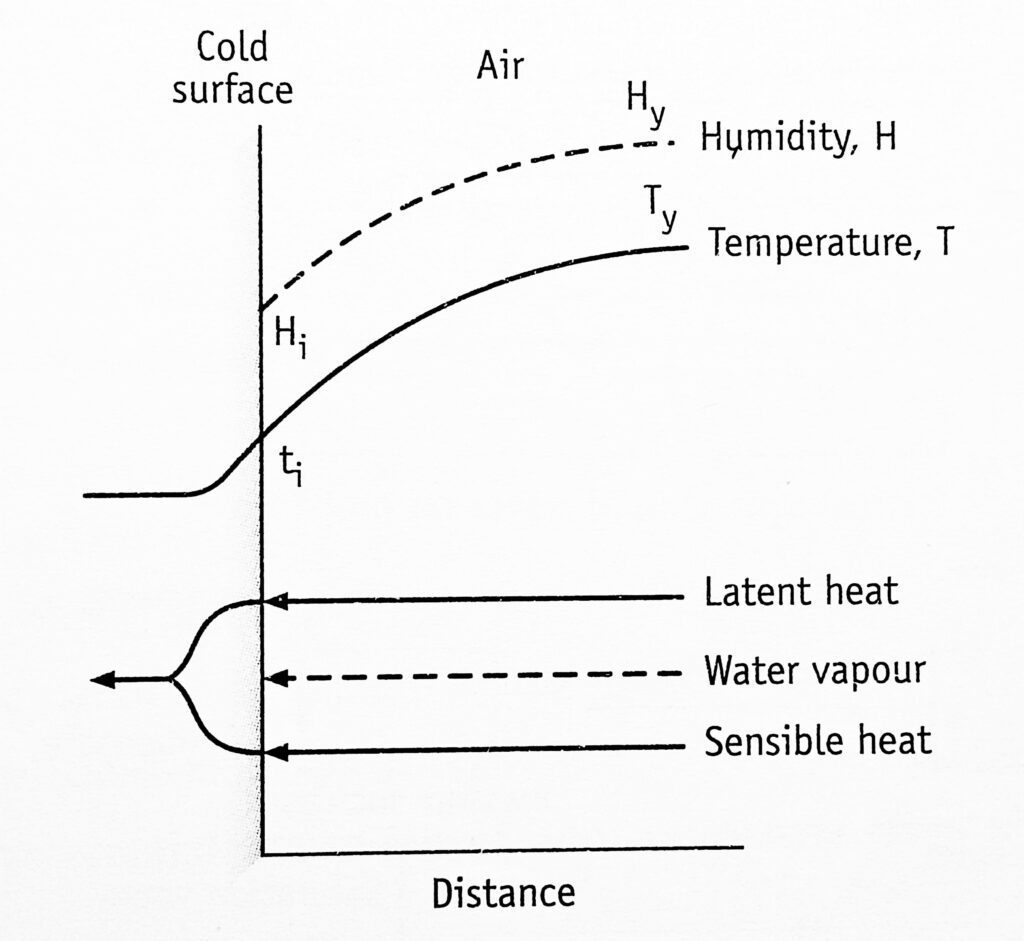
Since ti and hi represent saturated air, ty must be greater than ti Hence the bulk of the air would be saturated with water vapour at that temperature. The sensible heat is transferred into the water.
Approaches to Dehumidification
Different approaches to dehumidification are described in Fig. 3.
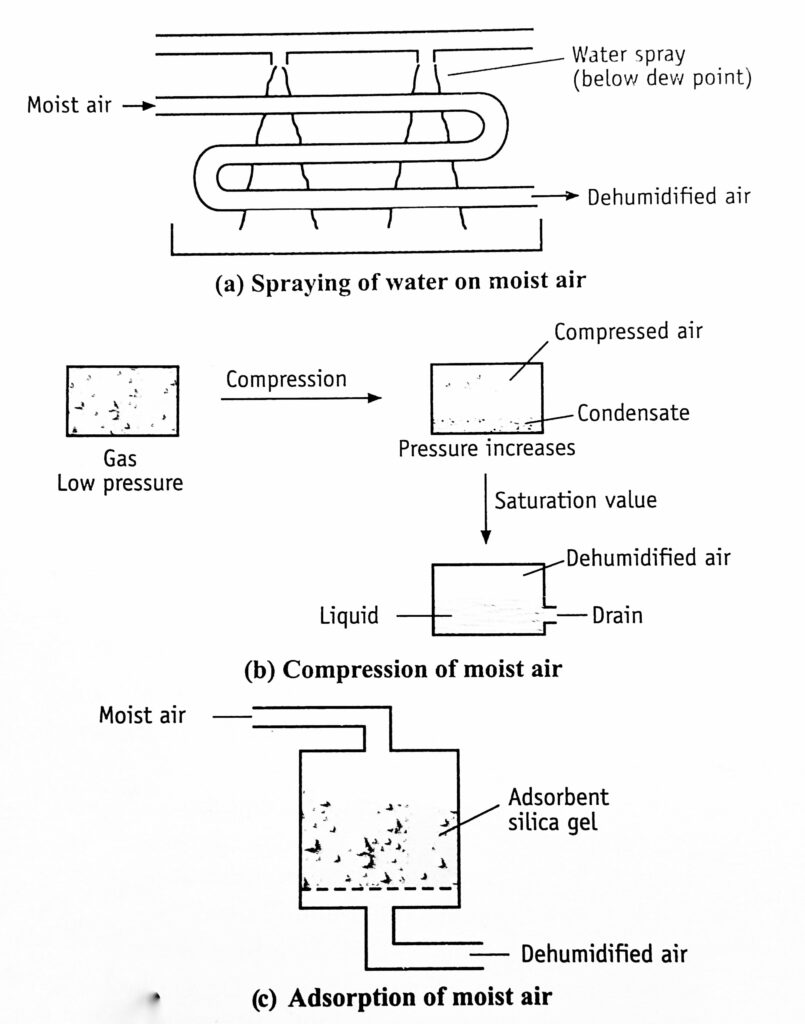
- Moist air is brought in contact with a spray of cold water, whose temperature is lower than the dew point of the entering air. (Figure 3a)
- Humidity can be reduced by compressing the air (Figure 3b). During compression, the partial pressure of the vapour increases. As soon as it reaches the saturation value, condensation takes place. The water gets liquefied and drained off (Figure 3c).
- Moist air is passed over a solid adsorbent surface or through a liquid adsorbent spray (Figure 10-9e). Dehumidification occurs due to the lowering of water vapour pressure. The adsorbents used are silica gel or activated alumina. The liquid adsorbents are solutions of inorganic salts (brine, lithium chloride) or organic compounds such as ethylene glycol. Reactivation of adsorbents is necessary. It is done intermittently or continuously. Silica gel has to be regenerated as soon as or even before it adsorbs 40% of its weight of water.
Suitable designs of equipment are evolved based on these principles.
Make sure you also check our other amazing Article on : Humidification