A reaction in which a particular stereoisomer reacts to give one specific stereoisomer of product is called a stereospecific reaction. If a reaction in which two or more stereoisomers are possible, but one stereoisomer is obtained more than the other is called a stereoselective reaction. Stereoselective and stereospecific reactions explain in below the reaction.
Stereospecific Reactions
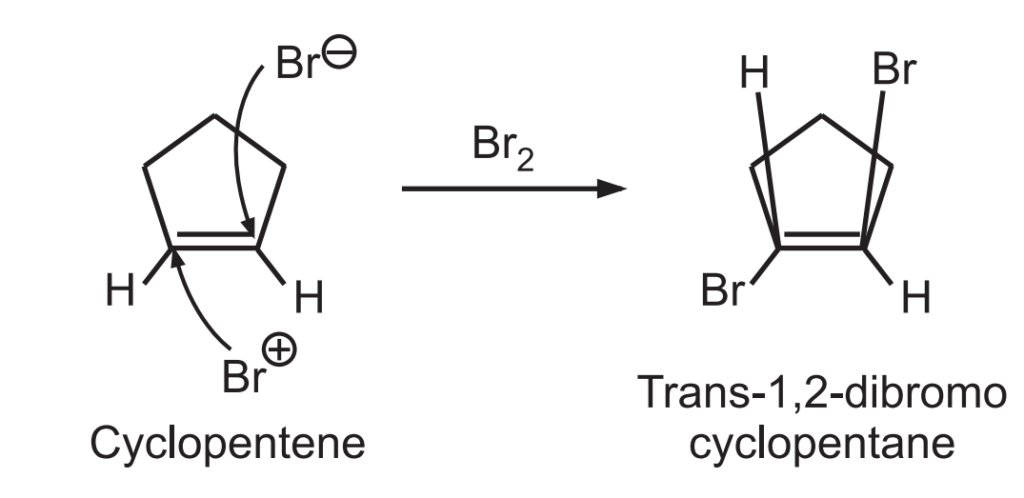
A stereospecific reaction is a reaction where the stereochemistry of the starting material governs the stereochemistry of the product. Only a single stereoisomer is produced in a given reaction rather than a mixture. For example, bromination of cyclopentene occurs through stereospecific anti-addition to give trans-1, 2-dibromocyclopentane only.
During the addition of dichlorocarbene to 2-pentene, the cis-2-pentene gives only one product, substituted cis-cyclopropane while the trans-2-pentene gives only one product, substituted trans-cyclopropane.
In yet another bromination reaction of 2-butene, two geometric isomers (cis and trans) of 2-butene give three stereoisomeric products where cis-2-butene gives (S, S) and (R, R) 2,3-dibromobutane while trans-2-butene gives meso-2,3-dibromo butane.
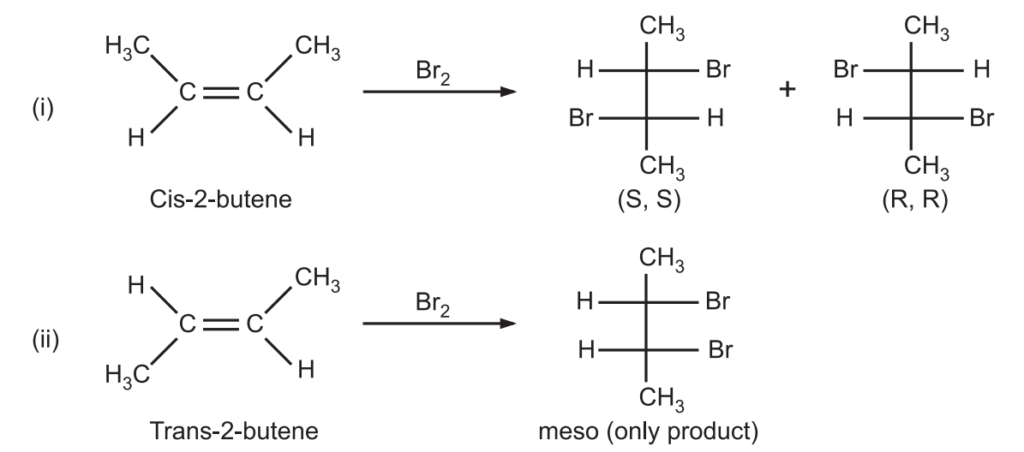
In the above case, bromination of cis-2-butene, the stereochemistry of products is governed by cis-2-butene. Here it is a stereospecific reaction. While bromination of trans-2-butene leads to the formation of only one product (meso). Hence, it is a stereoselective reaction.
Stereoselective Reactions
A stereoselective reaction is a reaction where one stereoisomer of a product is formed preferentially over another. If enantiomers of a chiral product are formed in unequal amounts, it is called an enantioselective reaction.
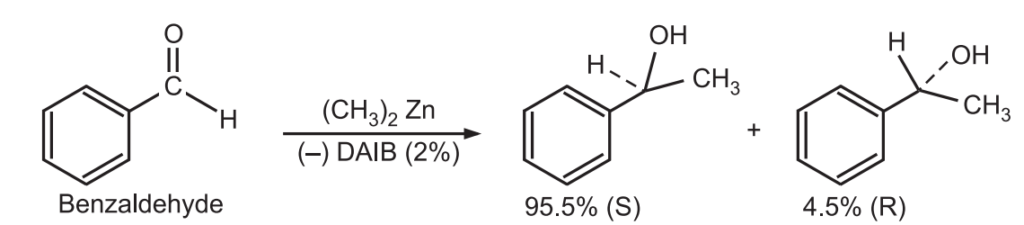
1. Enantioselective reaction:
2. Diastereoselective reaction:
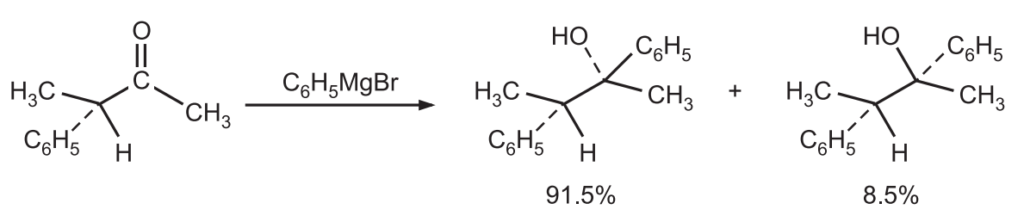
Similarly, when diastereoisomers are produced in unequal amounts, the reaction is called a diastereoselective reaction. In this reaction, two diastereoisomers could be formed but one is favored. All stereospecific reactions are stereoselective but stereoselective reactions are not necessarily stereospecific. For example, the reaction of HCl with propene gives 1-chloropropane and 2-chloropropane.
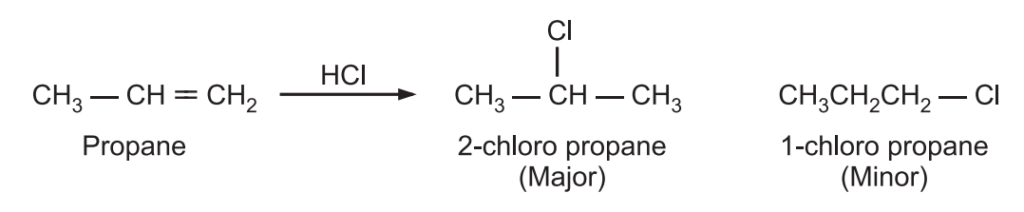
Since one product is favored over another, this reaction is said to be stereoselective. If the above reaction yields only 2-chloropropane, then the reaction is called stereospecific.
Make sure you also check our other amazing Article on : Meso Compounds