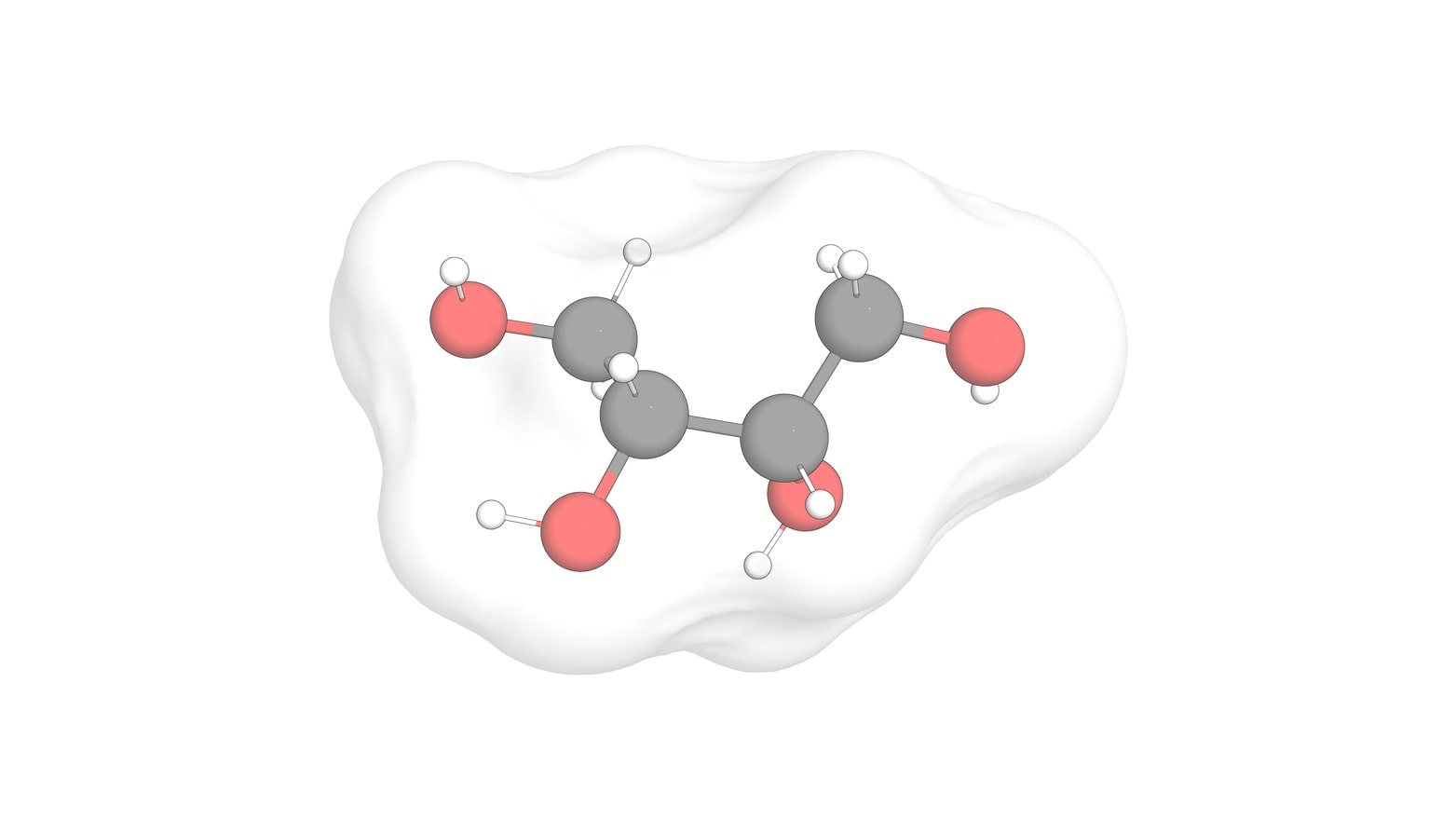
When multiple stereocenters present in a molecule create an internal plane of symmetry, it leads to meso compounds. Tartaric acid contains two asymmetric centers which give rise to four configurations. But there are really only three stereoisomers of tartaric acid: a pair of chiral molecules (enantiomers of each other) and the achiral meso compound. In meso compound, we have an internal mirror plane that splits the molecule into two symmetrical sides, the stereochemistry of both left and right sides should be opposite to each other. This leads to auto cancellation of stereo activity of each other resulting into optical inactivity. Hence, meso compounds can not be assigned with either dextrorotatory (+) or levorotatory (–) designation. The internal mirror plane is simply a line of symmetry that bisects the molecule. Each half is a mirror image of the other half. Each half must contain a stereocenter in order to be a meso compound. These stereocenters must also have different absolute configurations. Due to internal symmetry, the meso molecule is achiral. Hence, this configuration is not optically active. The meso form is also a type of diastereomer. The remaining two isomers are enantiomeric pair (D-and L-form).
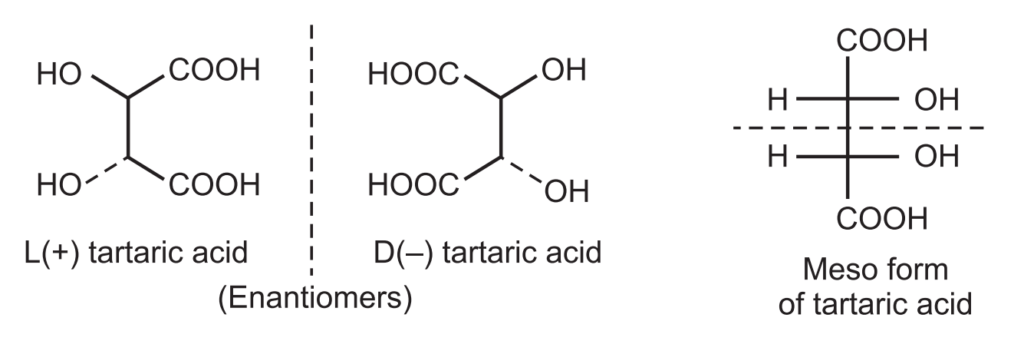
The melting point of both enantiomers of tartaric acid is about 170°C while the mesom tartaric acid has a melting point of 145°C. A meso compound is ‘superimposable’ on its mirror image. Examples in cyclic meso compounds include.
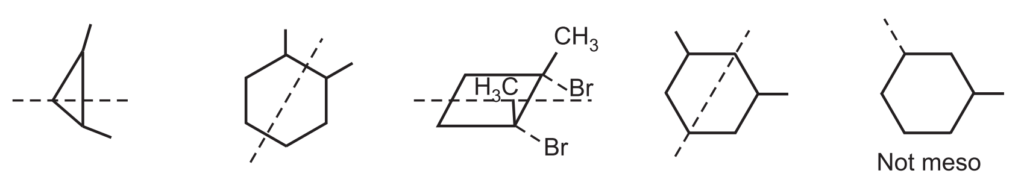
In summary, a meso compound should have two or more stereocenters, an internal symmetry plane and the stereochemistry should be R and S.
Make sure you also check our other amazing Article on : Geometrical Isomers