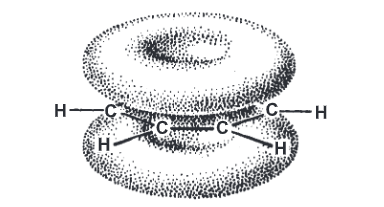
Aromatic Electrophilic Substitution: Benzene possesses a planar structure involving an sp² hybrid orbital of six carbon atoms. Each of these carbon atoms projects one unhybridized 2p atomic orbital at a right angle to the plane of the nucleus. The overlapping of ‘2p’ orbitals is extended over the whole system forming an x-orbital. A cloud of electrons thus exists above and below the plane of the benzene ring. This cloud protects the ring carbon atoms of benzene from the attack of nucleophilic reagents. Because of loosely held and easily available л electrons, the benzene ring serves as a base that can easily react with electron-deficient species. These reactions are called electrophilic aromatic substitutions.
Types of Aromatic Electrophilic Substitution
This category represents a variety of reactions like nitration, sulfonation, halogenation, Friedel Craft’s reactions, nitrosation, and diazo coupling. Unlike other reactions, nitrosation and diazo coupling need rings of high reactivity.
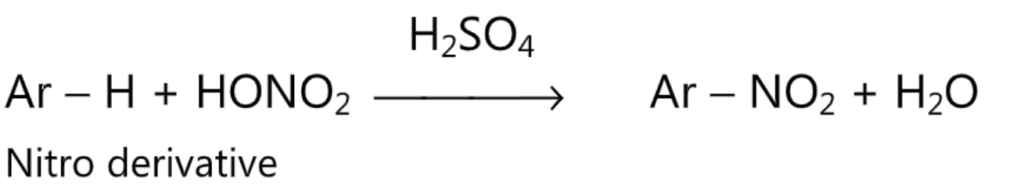
(1) Nitration:
(2) Sulphonation:

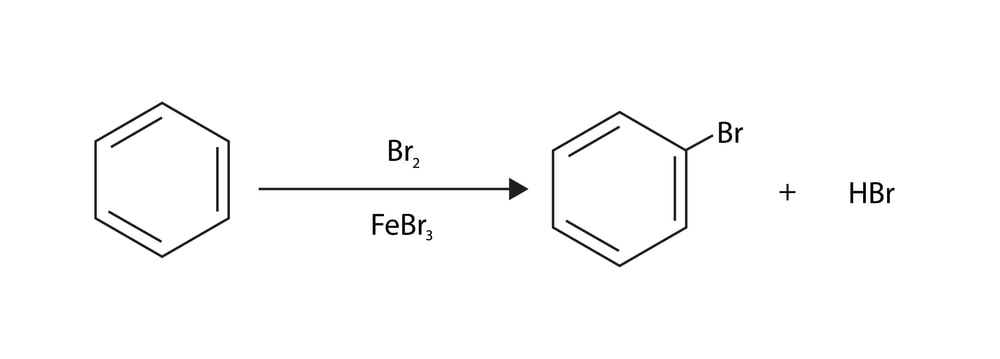
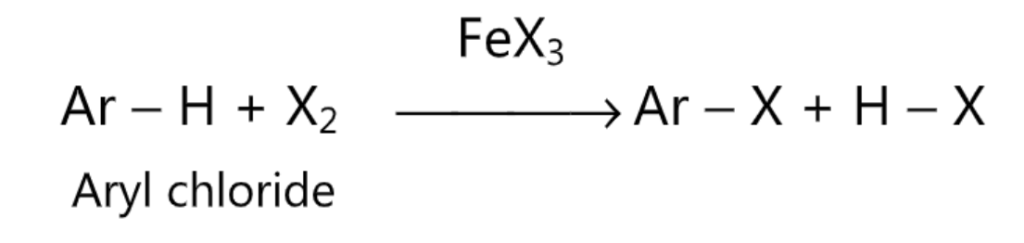
(3) Halogenation:
(4) FriedelCraft’s alkylation:
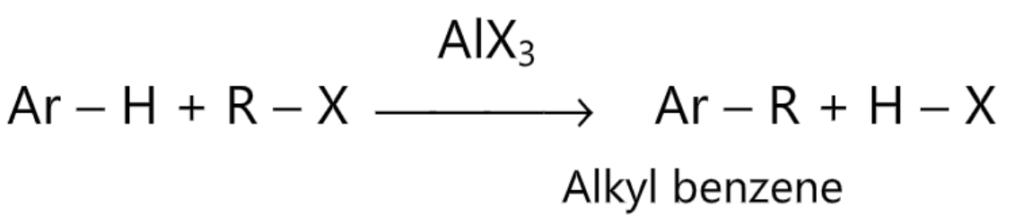
(5) Friedel-Craft’s acylation:
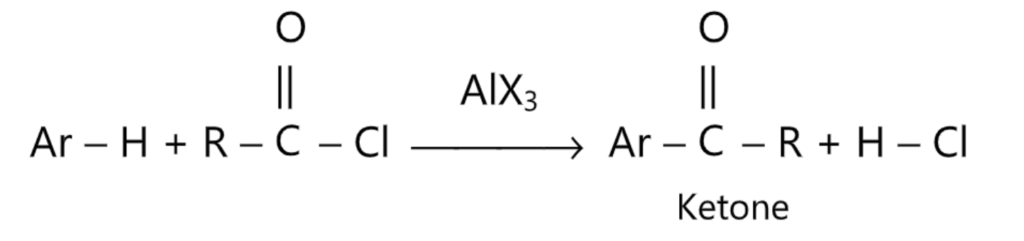
(6) Nitrosation:
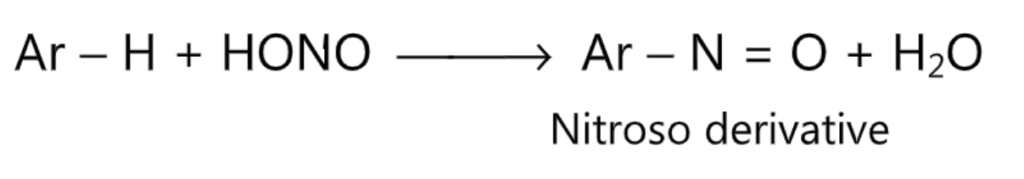
(7) Diazo coupling:
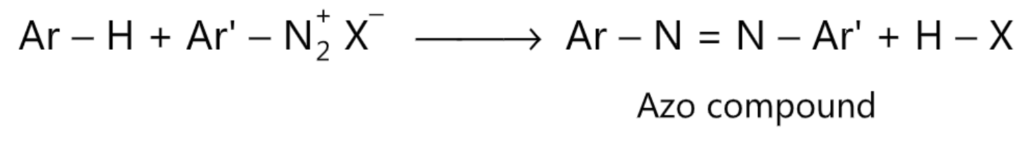
For better understanding refer this article Furan Reactions
Make sure you also check our other amazing Article on : Huckel’s Rule