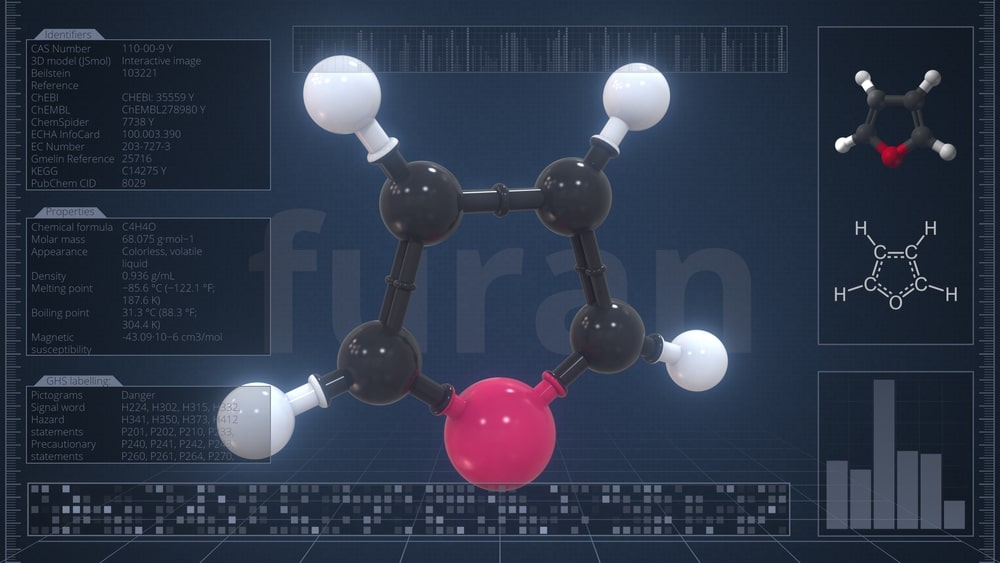
Furan is a π-excessive heterocycle and hence prefers electrophilic substitution reactions. At the same time, it behaves chemically as a typical diene and exhibits greater reactivity towards addition reactions.
Chemical Reactions of Furan
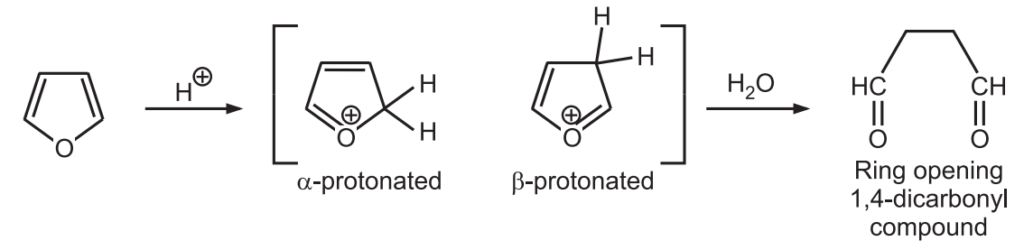
1. Protonation: Furans substituted with electron-withdrawing substituents are stable towards acid. However, the presence of electron-releasing substituents on furan leads to the generation of reactive electrophiles during protonation which activates polymerization and the ring-opening reactions.
2. Mercuration: Furan readily undergoes mercuration.
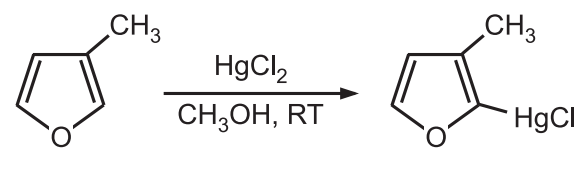
3. Reduction: Simple furan is difficult to reduce to a tetra hydro furan, without a ring-opening. Furoic acid can be reduced to a dihydro derivative.
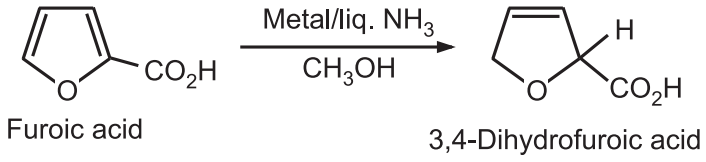
4. Electrophilic Substitution:
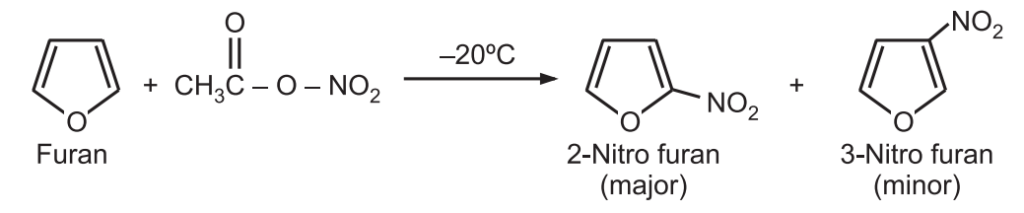
(a) Nitration: Furan is nitrated with a mild nitrating agent, acetyl nitrate, at a low temperature.
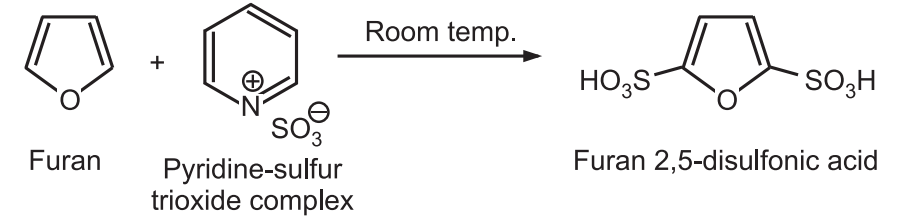
(b) Sulfonation: Furan is sulfonated with the complex of sulfur trioxide and pyridine or dioxane to give 2, 5 – disubstituted furan even at room temperature.
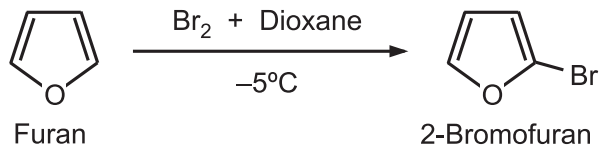
(C) Halogenation: The high reactivity of furan with chlorine and bromine at room temperature results in polyhalogenated products even at room temperature. Hence, milder conditions are required to yield mono-chloro or mono – bromo furans. Bromination of furan in DMF or dioxane at –5°C provides 2-bromofuran.
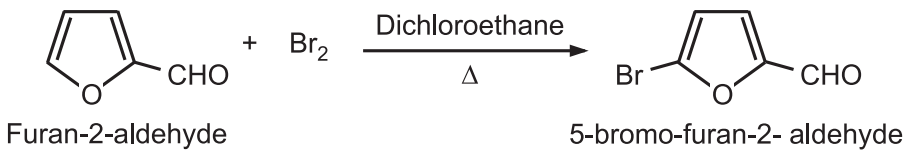
Furan substituted with an electron-withdrawing substituent at position-2 generally provides a 5-Bromo derivative.
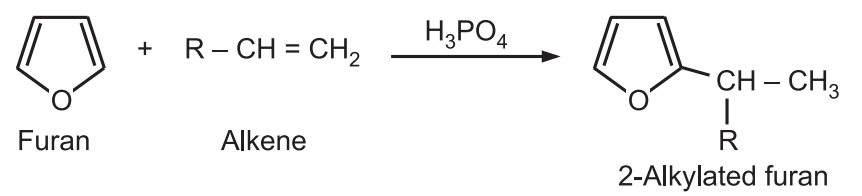
(d) Alkylation: Furan does not undergo Friedel-Crafts alkylation due to its acid sensitivity. Furan can be alkylated at position-2 using alkene in the presence of mild catalysts like phosphoric or boron trifluoride.
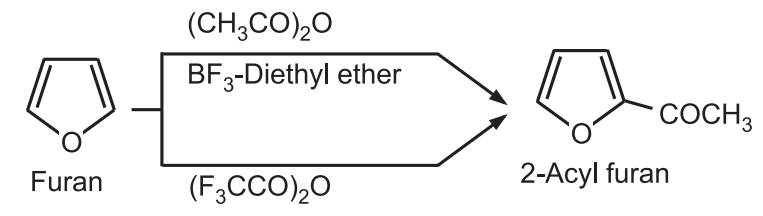
(e) Acylation: The acylation of furan with an acid anhydride or acid halides normally requires mild catalysts like phosphoric acid or boron trifluoride. No catalyst is required if trifluoroacetic anhydride is used as acylating agent.
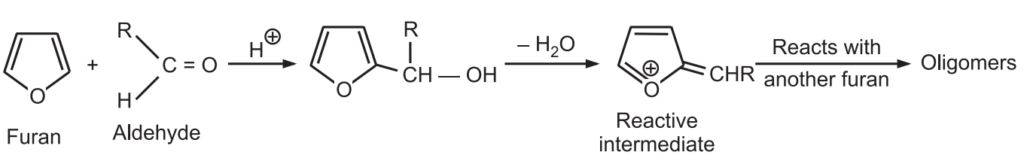
5. Condensation with aldehydes and ketones: In this acid-catalyzed reaction, furan condenses with aldehydes to produce a mixture of oligomers. A macrocycle is obtained by condensation of furan with acetone.
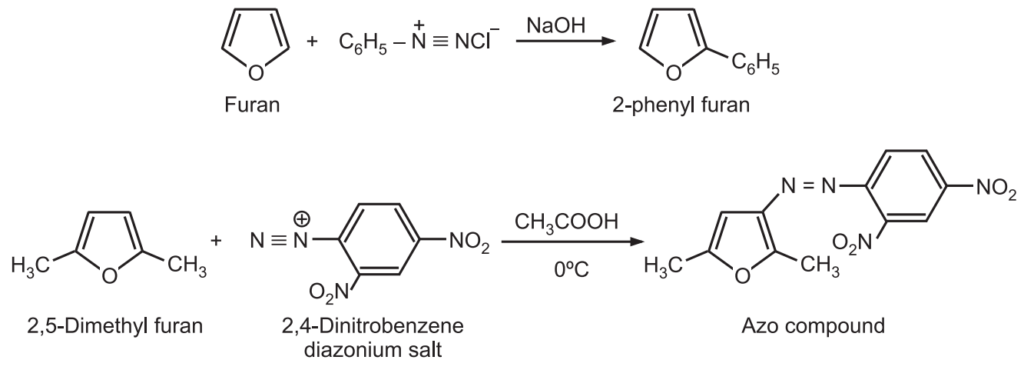
6. Reaction with diazonium salts: Furan reacts with a benzene diazonium salt to give 2-phenyl furan rather than the formation of an azo compound.
7. Reactions with nucleophilic reagents: Halofurans show more reactivity towards nucleophiles than simple furan. The presence of electron-withdrawing substituents (nitro, carboxy, or carboalkoxy) in halofurans further increases their reactivity.
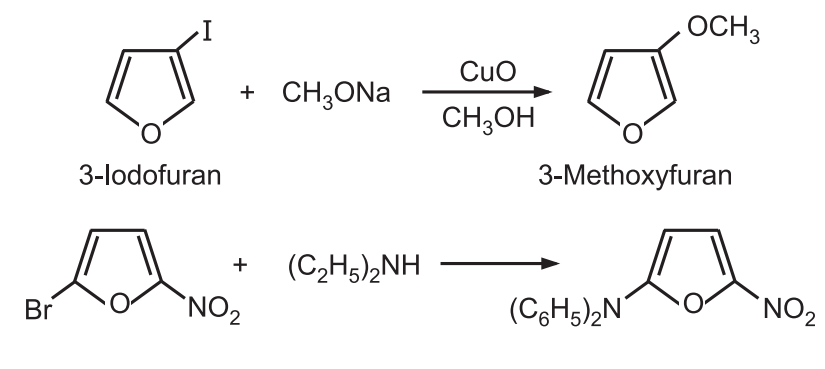
8. Oxidation: Ring-opening occurs when furan is treated with sodium hypochlorite, hydrogen peroxide, or meta chloroperbenzoic acid.
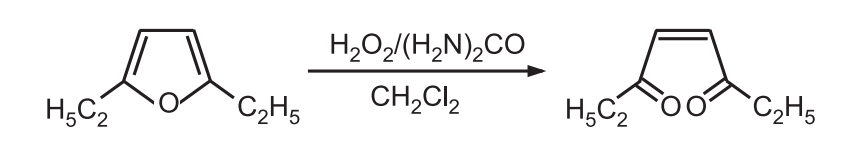
Medicinally Uses
Furan is an important scaffold present in drugs like ranitidine (anti-ulcer), nitrofurazone (anti-bacterial), ascorbic acid (vitamin C), and many natural terpenoids.
Make sure you also check our other amazing Article on : Furan Synthesis