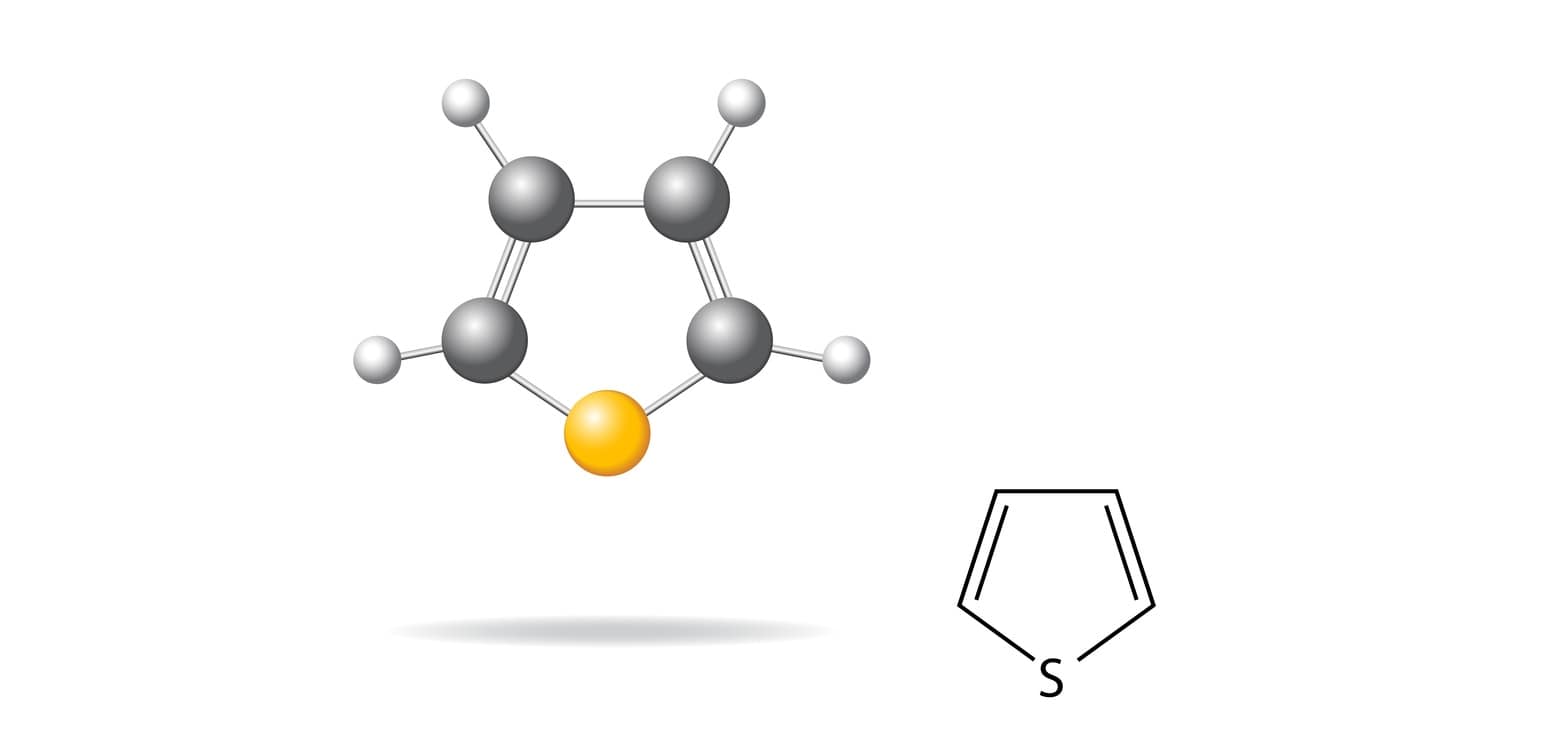
Thiophene Synthesis: Thiophene belongs to a class of heterocyclic compounds containing a five-membered ring made up of one sulfur as a heteroatom. Thiophene is a colorless liquid having a boiling point of 84°C. It was isolated as an impurity in commercial benzene in 1882 by Victor Meyer. It is a π – excessive aromatic heterocycle.
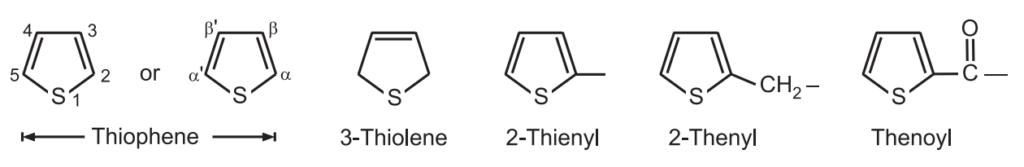
Chemical Synthesis of Thiophene
1. Paal-Knorr Synthesis: In this method, 1, 4 – dicarbonyl compounds can be heated with phosphorus pentasulfide (a source of sulfur) to give thiophene.
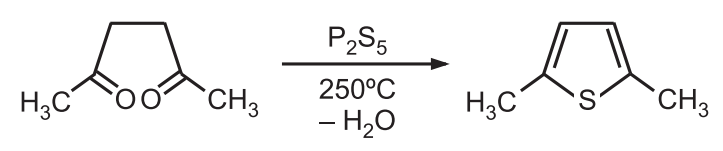
The basic mechanism of this synthetic procedure involves cyclic condensation of 1, 4-diketones (a) with primary amine (pyrrole synthesis), (b) with a sulfur source (thiophene synthesis), or (c) dehydration of diketone (furan synthesis). Phosphorus pentasulfide or bis (trimethylsilyl) sulfide acts as a sulfurizing agent as well as a dehydrating agent. Hydrogen sulfide in the presence of an acid catalyst is also effective.
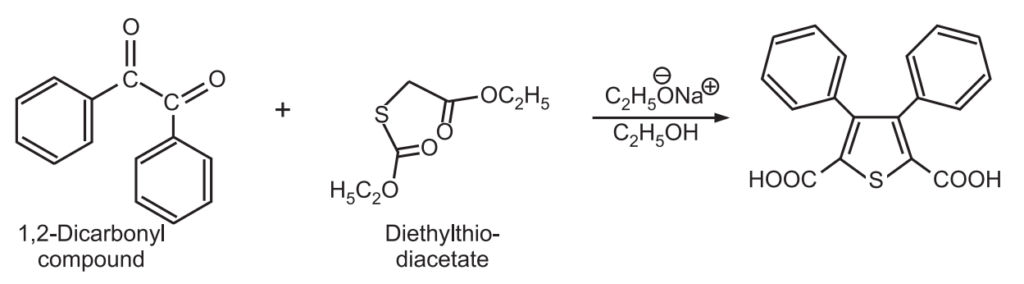
2. Hinsberg Synthesis: Two consecutive aldol condensations between 1, 2-dicarbonyl compound and diethylthiodiacetate in the presence of a strong base gives thiophene.
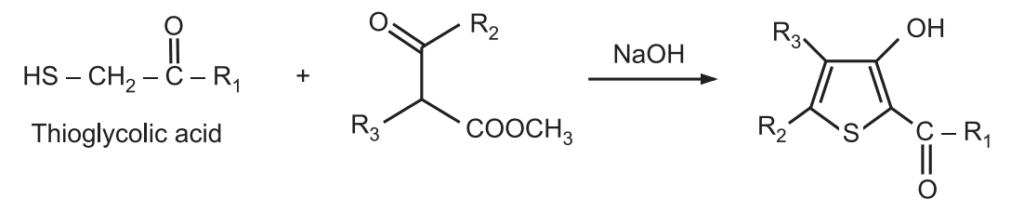
3. Fiesselmann Thiophene Synthesis: It is a base-catalyzed condensation reaction of thioglycolic acid with α, and β-acetylenic esters to give 3-hydroxy-2-thiophene carboxylic acid.
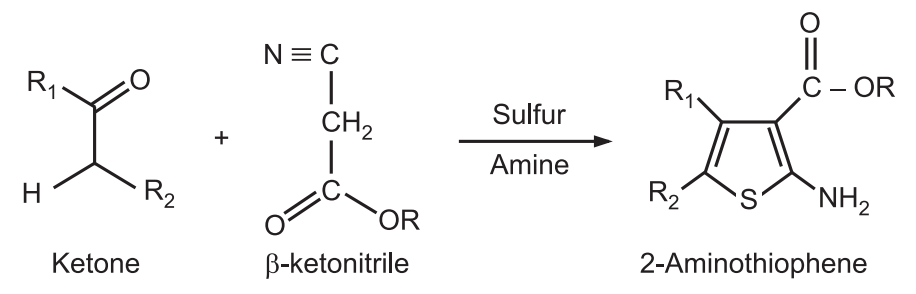
4. Gewald Aminothiophene Synthesis: It is a base-catalyzed condensation of a ketone with a β–acetonitrile to form an olefin, followed by cyclization with elemental sulfur to give 2-aminothiophenes.
5. Industrial Methods:
(1) Thiophene can be synthesized on an industrial scale heating n-butane and sulfur at high temperatures.
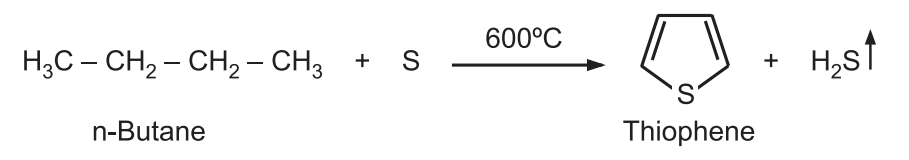
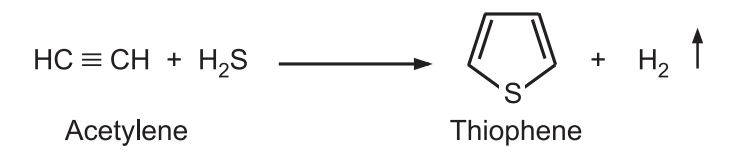
(2) Using n-butane the sulfur first causes dehydrogenation and then interacts with the alkene by addition. Further dehydrogenation leads to the aromatization of the ring. A mixture of acetylene and hydrogen sulfide is passed through a tube containing alumina at 400°C.
(3) A mixture of sodium succinate and phosphorus trisulfide is heated at 200°C to give thiophene.
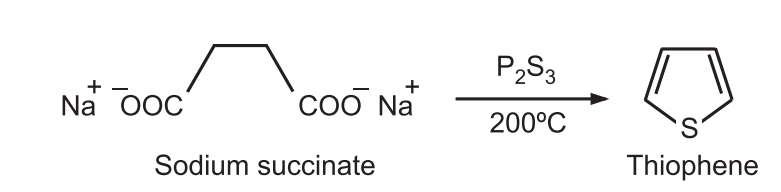
Make sure you also check our other amazing Article on : Furan Reactions