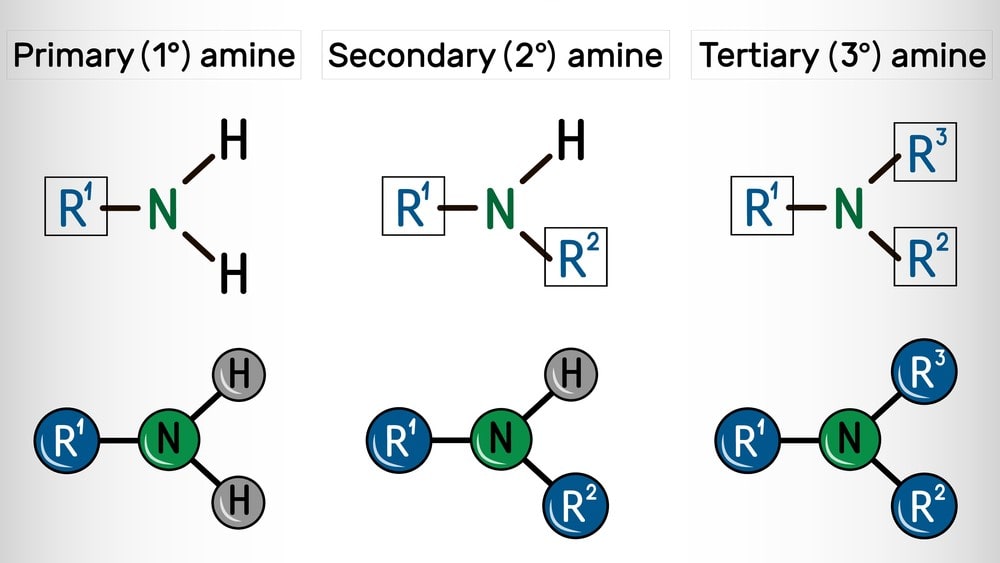
The principle reaction of amines are related to the replacement of -H from the nitrogen atom; thus they are limited to primary and secondary amines.
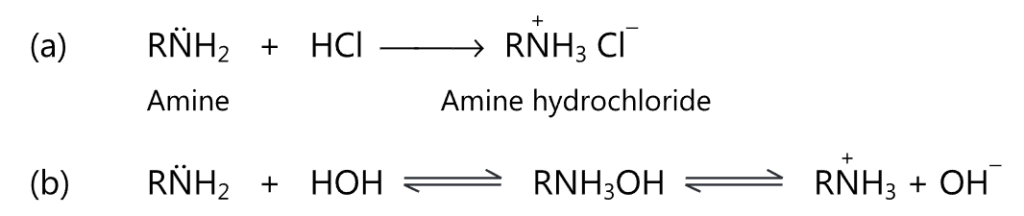
All the amines are basic due to the presence of a lone pair of electrons on the nitrogen atom which may be used for protonation. For example,
(c) When heated at high temperature, an amine salt decomposes to give a molecule of alkyl halide.
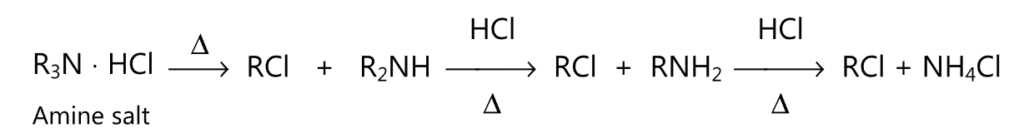
Table of Contents
(A) Reactions are shown by primary amines:
(1) When heated with ethanolic potassium hydroxide and chloroform, primary amine yields isocyanide.
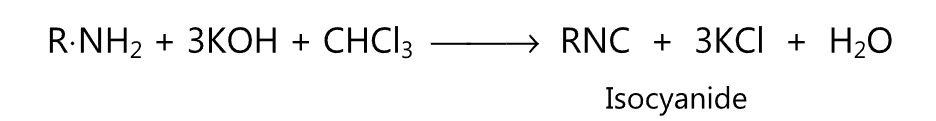
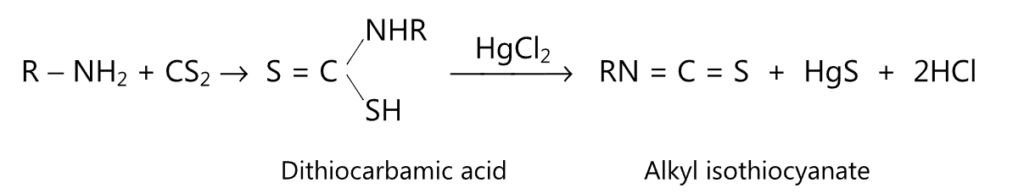
(2) Hofmann mustard oil reaction: When warmed with carbon disulfide, primary amine forms a dithiocarbamic acid. The latter gives alkyl isothiocyanate upon decomposition by mercuric chloride.
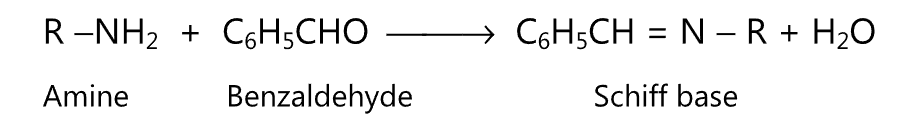
(3) Schiff base is formed when primary amine reacts with an aldehyde.
(4) An alcohol is obtained when primary amine reacts with nitrous acid.
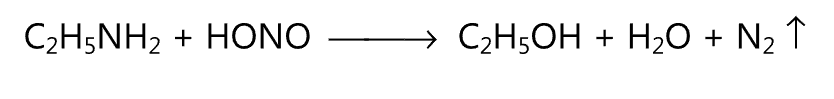
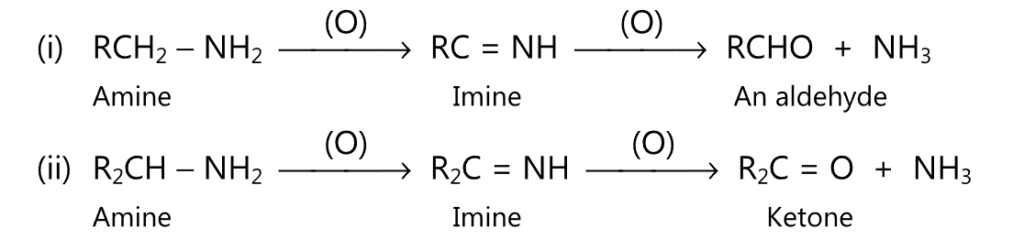
(5) Upon oxidation by potassium permanganate, the primary amine may give an aldehyde or a ketone.
(B) Reactions are shown by secondary amines:
(1) A dithiocarbamic acid formed due to the reaction of a secondary amine with carbon disulfide, does not undergo decomposition by mercuric chloride.
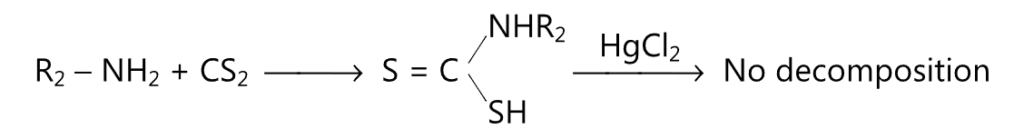
(2) Schiff base is formed when secondary amine reacts with an aldehyde containing α-hydrogen.
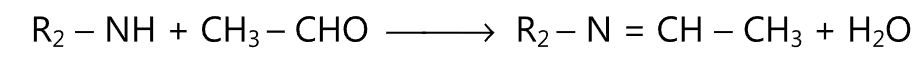
(3) A diamine is obtained upon oxidation of secondary amine by potassium permanganate.
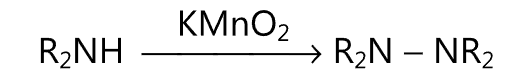
(4) Secondary amine reacts with nitrous acid to form insoluble oily nitrosamine. Nitrogen gas is not evolved.
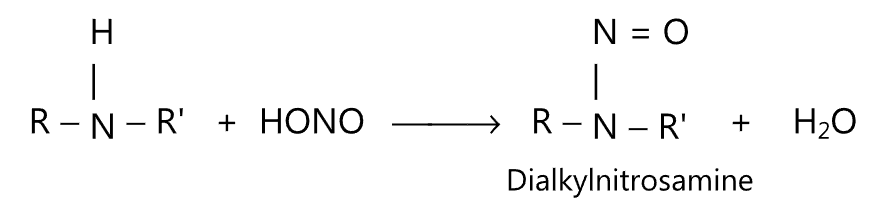
This reaction is the basis of Libermann’s nitroso reaction.
(C) Reactions are shown by tertiary amines:
(1) Potassium permanganate does not cause oxidation of tertiary amine. However, hydrogen peroxide oxidizes later to give amine oxide.

(2) A nitrite salt is obtained when the tertiary amine is treated with nitrous acid.

(3) When tertiary amine is treated with cyanogen bromide, a dialkyl cyanamide is obtained. The latter upon hydrolysis with acid or alkali gives a secondary amine.
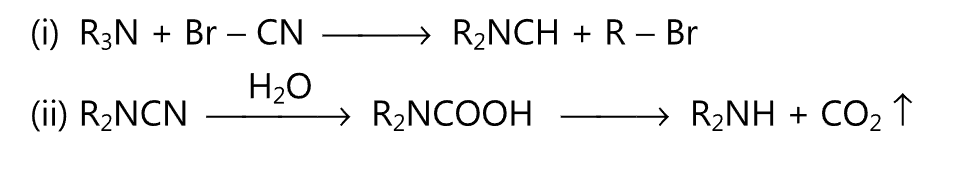
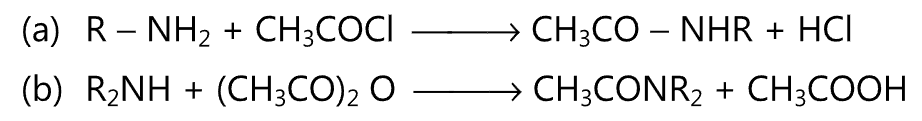
(D) Reactions are shown by primary and secondary amines:
(1) Amines react with acid chlorides or anhydrides to give N-acyl amide. For example,
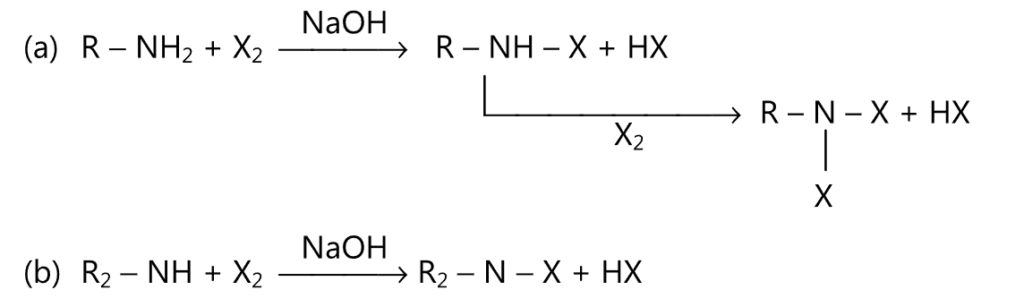
(2) Mono or dihalide derivatives are obtained when amines are treated with halogen in the presence of alkali.
(3) Upon treatment with nitrosyl chloride, primary amine gives alkyl chloride while secondary amine gives nitrosamine.
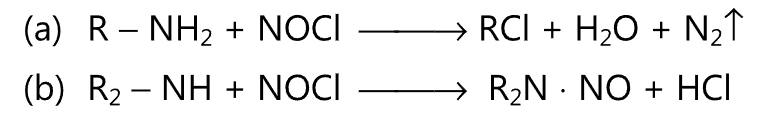
(4) Primary amine reacts with Grignard reagent as follows:
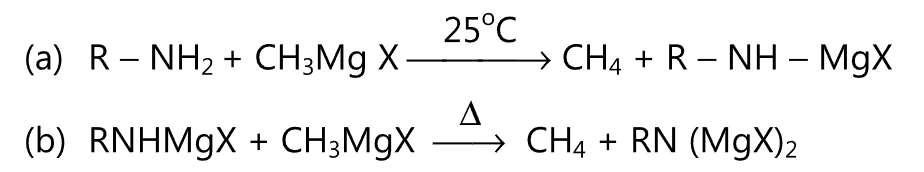
While secondary amine reacts with only one molecule of a Grignard reagent.
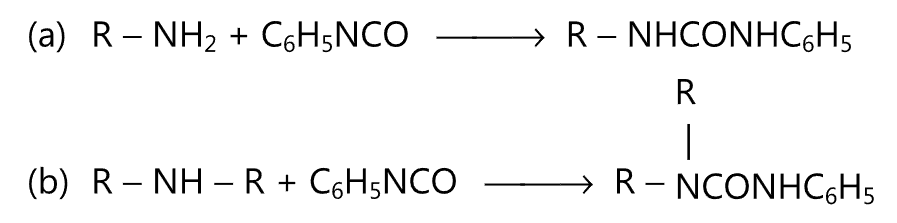
(5) Substituted ureas are formed when amines are treated with phenyl isocyanate.
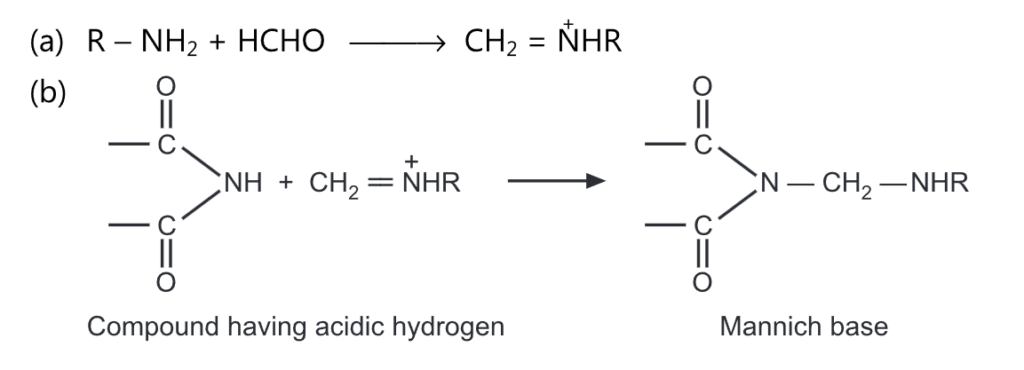
(6) Mannich reaction is reported to occur when a compound containing acidic hydrogen is treated with amine and formaldehyde.
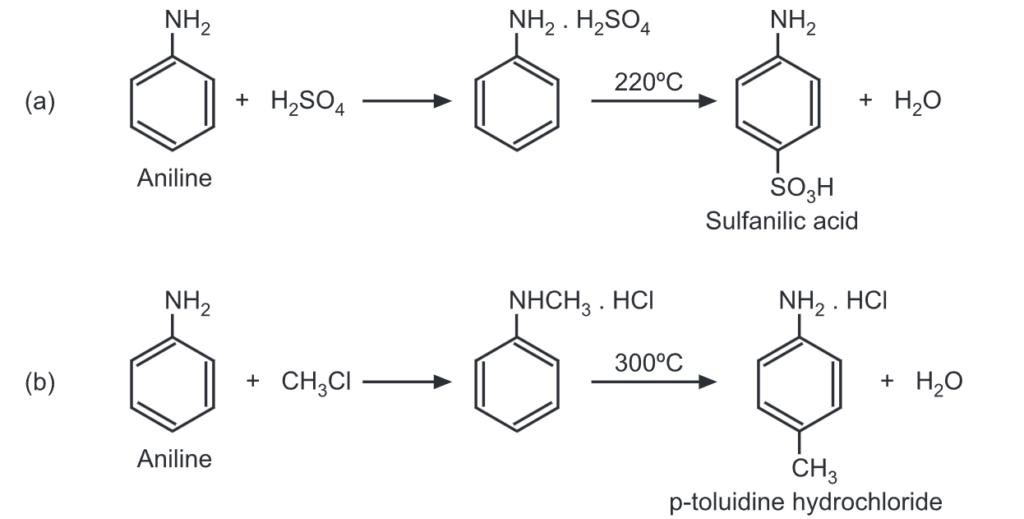
The reactions of aromatic amines are centered around the amino group, with the amino group generally being the point of the initial attack. The product obtained may then rearrange usually at high temperature to give a compound with a ring substitution releasing the free amino group.
Make sure you also check our other amazing Article on : Preparation of Amines