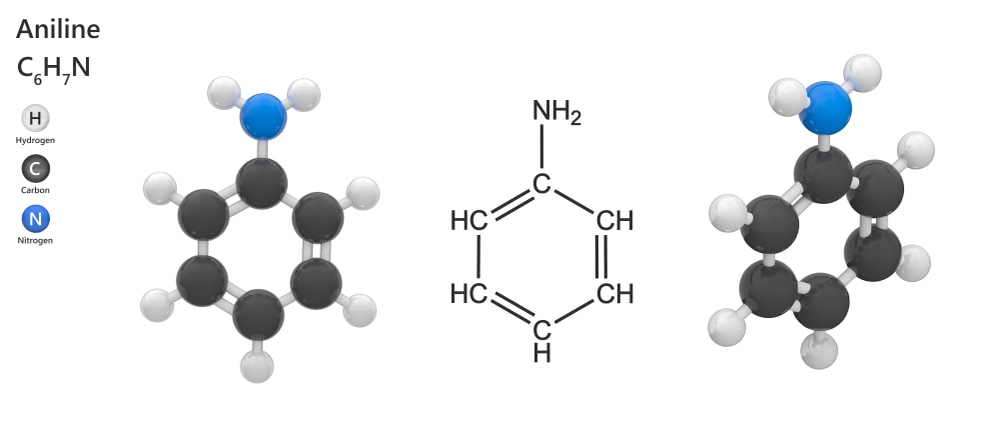
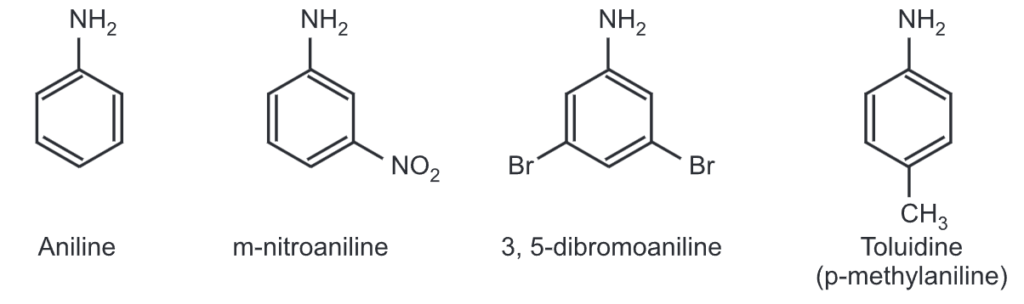
Aromatic amines are named derivatives of aniline.
In the salts of amines, the positive charge is present in the nitrogen. This cation is called as ammonium (or anilinium in aromatic amines). The salt is named by prefixing ammonium to the name of the anion. For example,
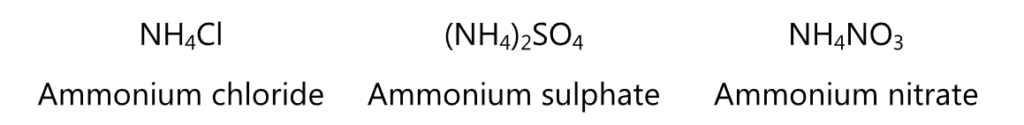
Because of their polar nature, amines can form intermolecular hydrogen bonds. Hence, they have higher boiling points than non-polar compounds having the same molecular weight. Amines are basic in nature. If exposed to air, aromatic amines (Ar – NH2) are readily oxidized to Ar- NO2.
Make sure you also check our other amazing Article on : Structure and Uses of Phenol