The structure and uses of Phenol:
(1) Phenol: It is used as an antiseptic and disinfectant. It is also used as a starting material to make plastics, explosives (e.g. picric acid) and drugs (e.g. aspirin). Substituted phenols are used in dye industries to make azo dyes.
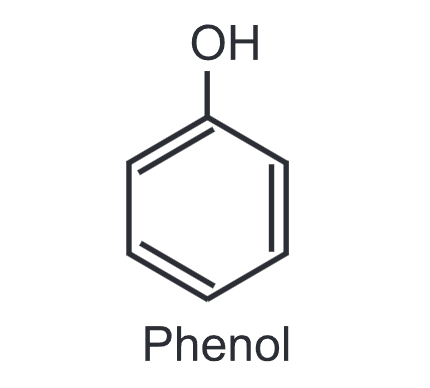
Phenolic resins are extensively used in electric switches and automobiles due to their property of withstanding extreme conditions of heat and resistance to electricity. Phenol is also used in the cosmetic industry in the manufacturing of sunscreen and hair colouring solutions.
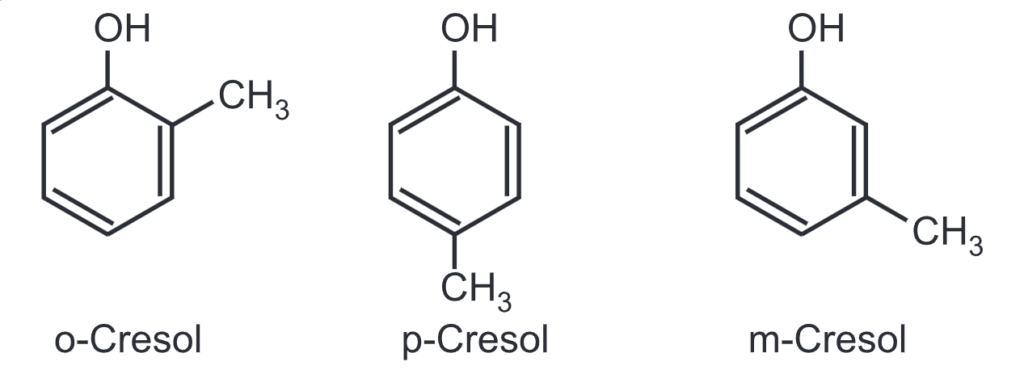
(2) Cresol (Methyl phenols): There are three forms of cresol. The mixture of all three forms is known as tricresol. Cresols are precursors used in plastics, dyes and pharmaceutical industries. p-Cresol is mainly used in the production of antioxidants like butylated. hydroxytoluene (BHT).
(3) Resorcinol (m-dihydroxybenzene): It is a colourless needle-shaped compound easily soluble in water, alcohol and ether. Externally, it is used as an antiseptic and disinfectant.
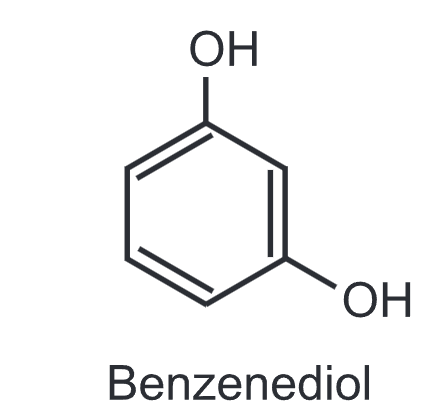
It is also used in 1-2% concentration topically in acne treatment. It can be used as an anti-dandruff agent in shampoo or in sunscreen lotions. It is used internally in doses of 125-250 mg in the treatment of gastric ulcers.
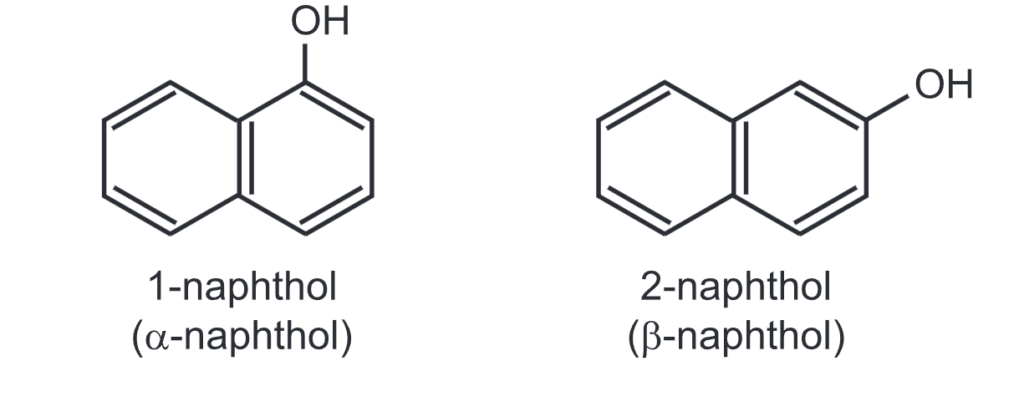
(4) Naphthols: Naphthols are fluorescent white/colourless compounds, a-naphthol is a precursor to a variety of insecticides and pharmaceuticals. Besides naphthol undergoes azo coupling to give various azo dyes…
Make sure you also check our other amazing Article on : Reaction of Phenols
