Phenols are organic compounds containing a benzene ring bonded to a hydroxyl group.
The acidity of phenols: Phenols are more acidic than alcohol but less acidic than carboxylic acids. The acidity of phenol is due to its ability to lose hydrogen ions to form phenoxide ions. Phenol is a weak acid. For example,
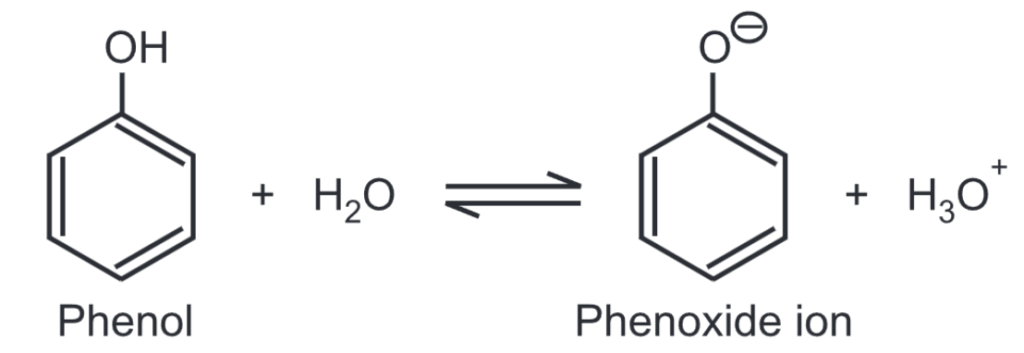
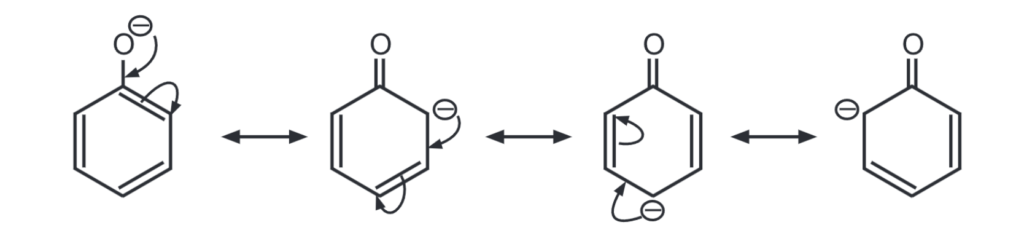
The negative charge on the oxygen atom is delocalized around the ring. As a result, the negative charge is no longer entirely localized on the oxygen but is spread out around the whole ion.
The negative charge of the phenolate ion is stabilized by resonance. This makes the phenoxide ion more stable.
Acidic characteristics of phenols:
(i) pH of a dilute solution of phenol in water ranges between 5-6.
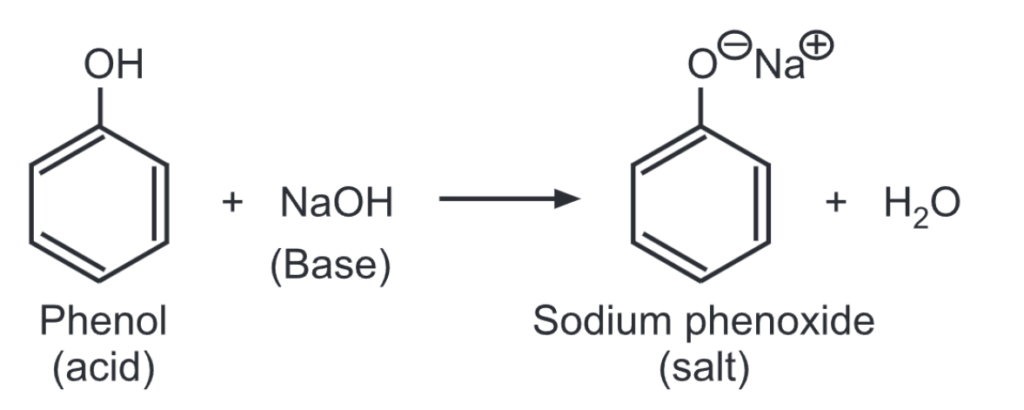
(ii) Phenol reacts with sodium hydroxide solution to give a colorless solution containing sodium phenoxide.
(iii) Due to its weak acidic nature, phenol partially reacts with sodium carbonate to give sodium phenoxide and sodium bicarbonate.
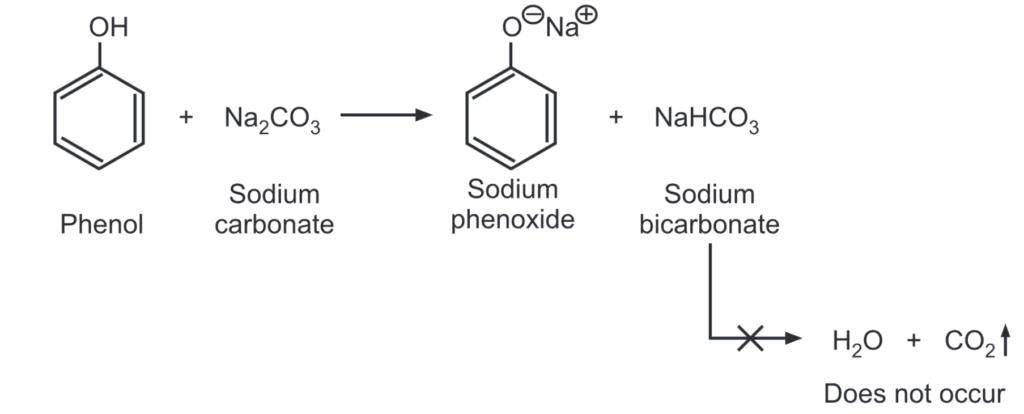
Unlike other carboxylic acids, phenol is not acidic enough to react with sodium bicarbonate to produce carbon dioxide and water.
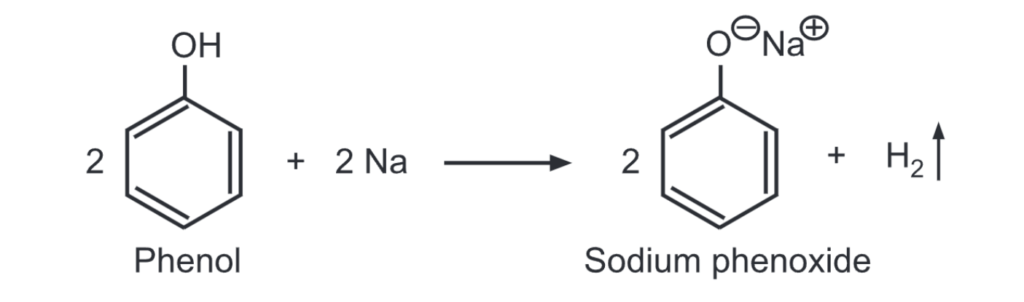
(iv) Like other acids, phenol reacts with metallic sodium/potassium to give hydrogen gas. Phenol being a weak acid is a slow reaction.
Effects of Substituents on Acidity:
The resonance structures of phenoxide ion explain the delocalization of negative charge at ortho and para positions of the benzene ring.
Substituents, particularly those located ortho or para to the -OH group, can dramatically influence the acidity of the phenol due to resonance and/or inductive effects.
Electron withdrawing groups like -NO2, -COOH increase the stability of phenoxide ions.
Table 1.1: Effects of Substituents on the acidity of phenol
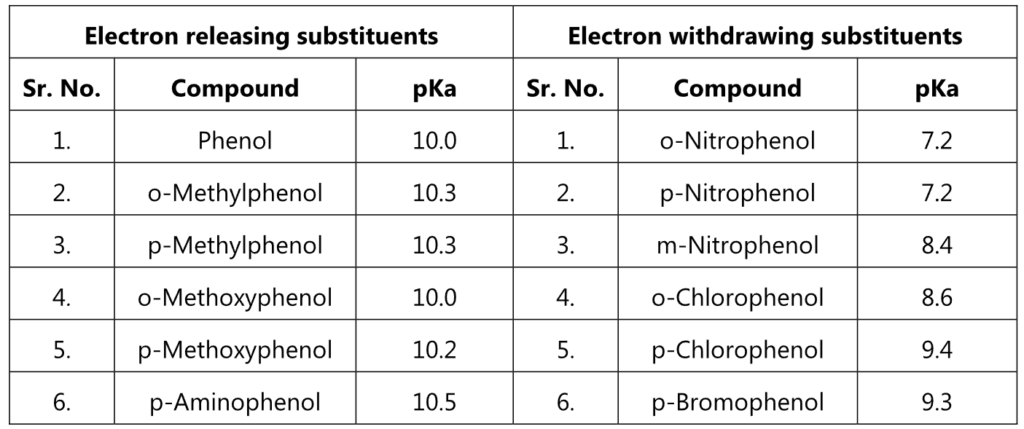
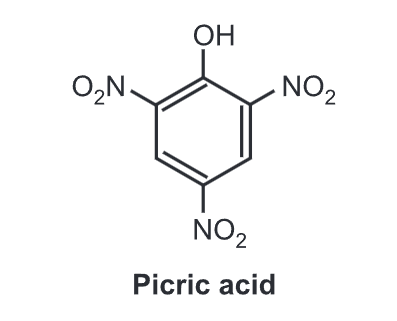
Hence, these groups increase the acidity of phenols, if attached at the ortho or/and para positions to the hydroxyl group. For example, the influence of a nitro substituent is over ten times stronger in the para-location than in meta. Additional nitro groups have an additive effect if they are positioned at ortho and para positions. For example, 2,4,6-trinitrophenol (picric acid) is a very strong acid.
While electron donating groups like – CH3, – OH, -NH2 decrease the acidity of phenols as they prohibit the formation of phenoxide ions.
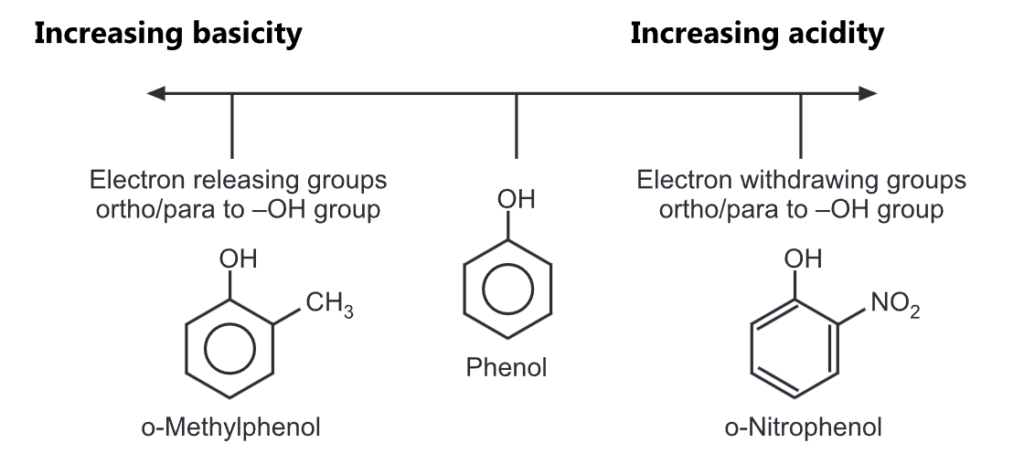
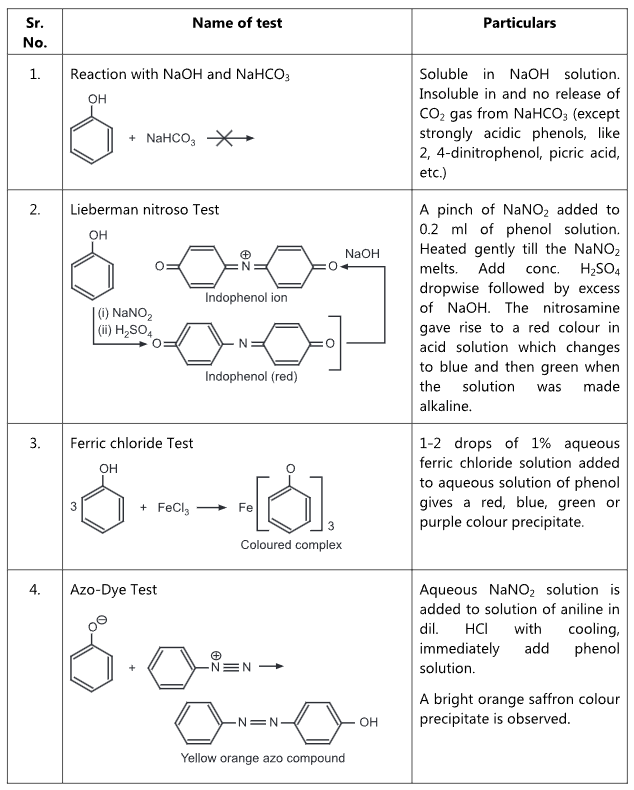
Table 1.2: Qualitative tests for Phenols
Make sure you also check our other amazing Article on : Diazo Coupling Reaction