Conformational Isomerism is the isomers generated due to rotation around single bonds present in the molecule. They may be rapidly interconverted to each other again through further rotation around single bonds. There exists a rotational energy barrier that needs to be overcome to convert one conformer to another.
Some important examples of conformational isomerism include:
- Open chain alkane conformations: Staggered, eclipsed and gauche conformers.
- Ring confirmation: Chair and boat conformers.
- Atropisomerism: A molecule can become chiral due to restricted rotation about a bond.
- Folding of the molecule.
Conformations of Ethane
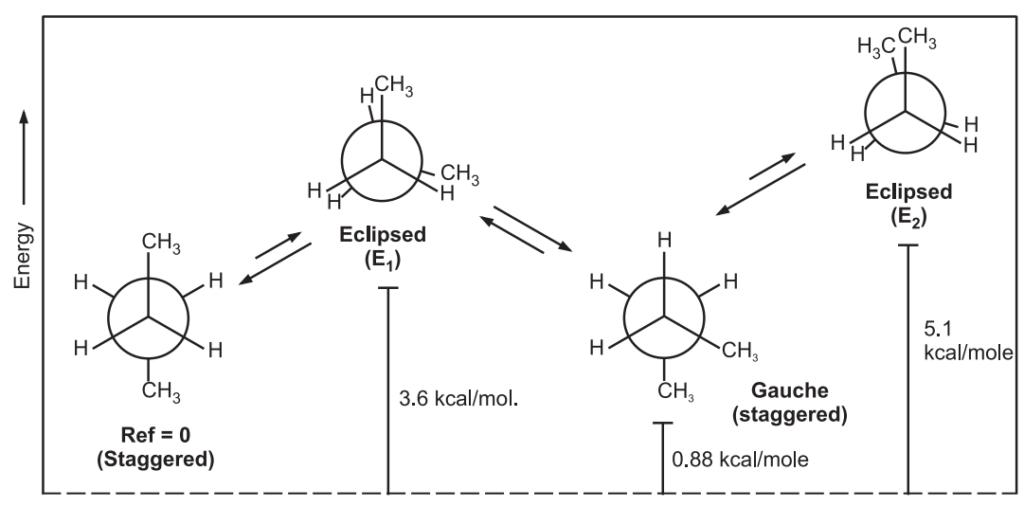
Out of the infinite conformations possible, the most important conformers of ethane are: (i) Staggered conformation and (ii) Eclipsed conformation.
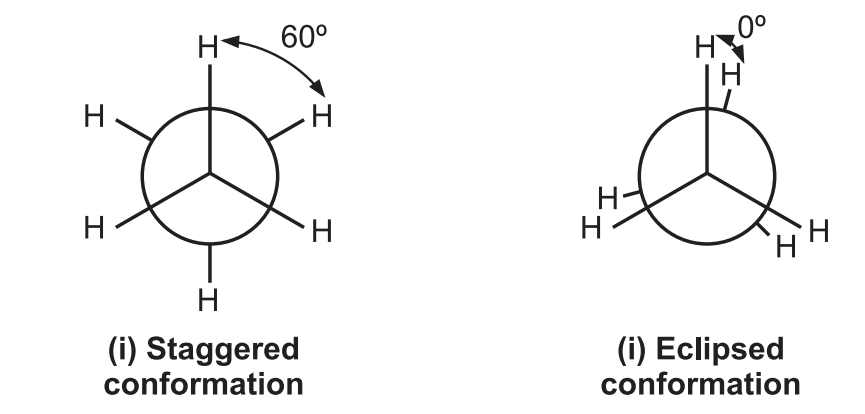
Since the angle between the C – H bonds of the 1st and 2nd carbons is 60º, the staggered conformation is the most stable conformation of ethane. In eclipsed form, the angle is 0º between two C – H bonds leading to repulsion in their electron cloud which raises the energy and decreases the stability of the molecule. The eclipsed conformation of ethane is less stable than the staggered conformation by 3 kcal/mol.
In eclipsed conformation, the bulky substituents of the molecule are brought closer leading to repulsion among them. This hindrance causes resistance to rotation (torsional strain i.e., a force that opposes rotation due to the repulsion of bonding electrons.) It is not possible to isolate either of the ethane conformations due to their rapid interconversion at room temperature.
Conformations of n-Butane
Various conformations of n-Butane include,
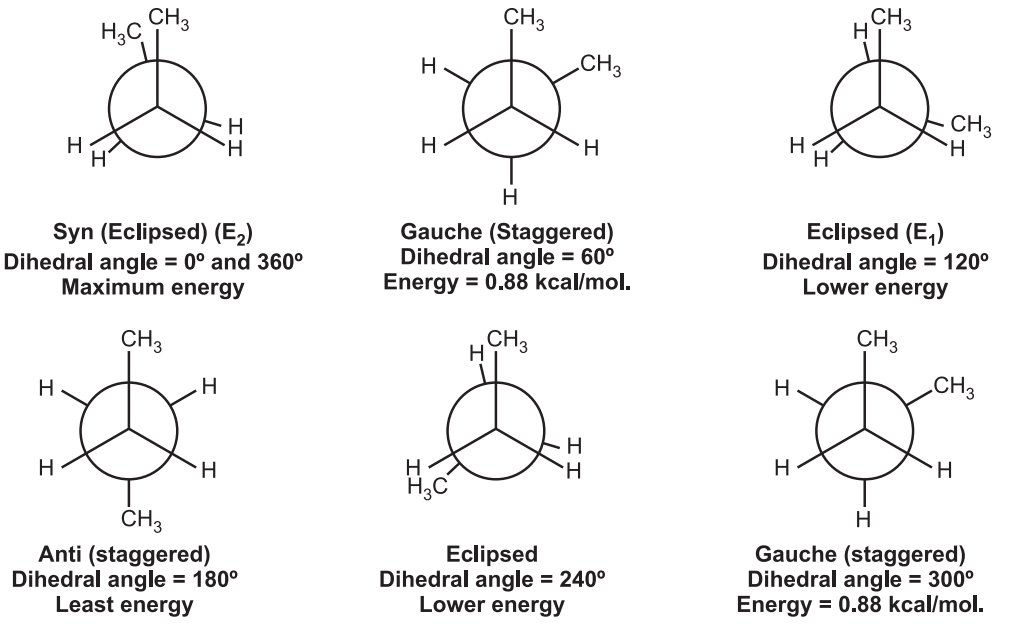
Dihedral angle: It is the angle created by two intersecting planes.
Table 1.1: Types of interactions in conformers of n-butane
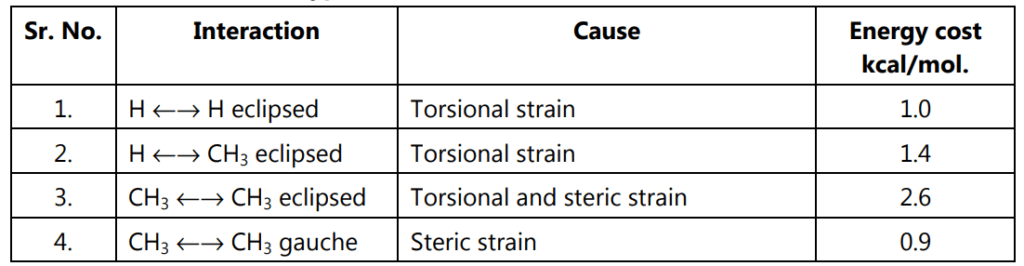
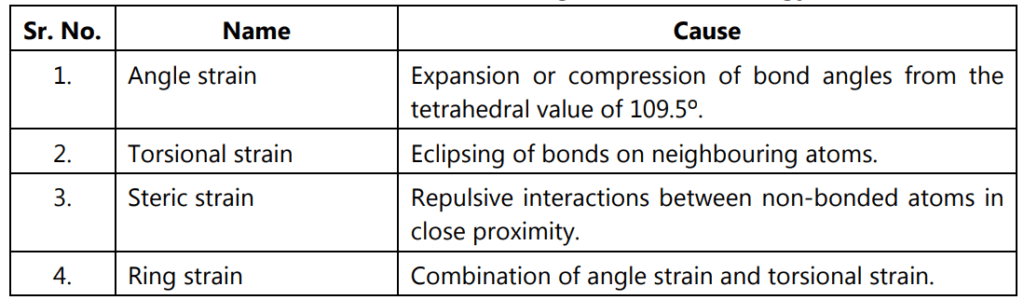
Table 1.2: Various strains contribute to the rotational energy barrier
The gauche butane is less stable than antibutane by 3.8 kJ/mol. because of steric interference between H-atoms on the two methyl groups.
Conformations of Cyclohexane
In cyclohexane, all carbon atoms are sp3 hybridized with a bond angle of 109º. This leads to two types of conformations.
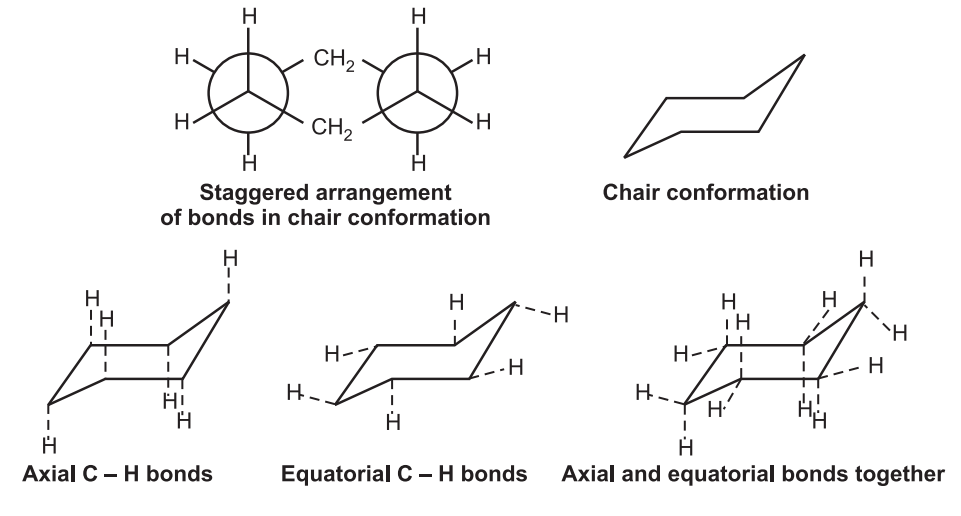
1. Chair conformation: It is the most stable form having a tetrahedron bond angle of 109º. It adopts a staggered arrangement having the least torsional strain.
Chair cyclohexane has six axial hydrogens perpendicular to the ring (parallel to the ring axis) and six equatorial hydrogens near the plane of the ring.
Six-membered rings are almost free of the strain in a chair conformation.
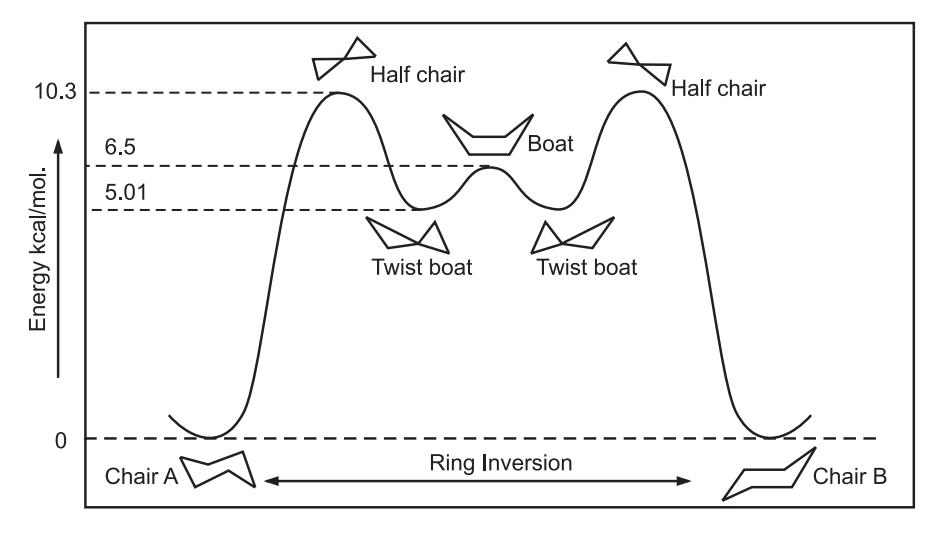
Boat form can be obtained from chair conformation by bending the bonds. This transformation of chair to boat form occurs through the intermittent – half-chair and twist-boat form. In boat conformation, carbon 1 and 4 are bent towards each other while all hydrogens in the chair conformation are staggered, four hydrogens are eclipsed in the boat conformation. Hence, the boat conformation is less stable than a chair conformation by 6.5 kcal/mol.
As a result of simultaneous rotation about all C – C bonds, chair conformations readily get interconverted, resulting in the exchange of axial and equatorial positions. It is known as ring inversion or ring flip. In this process, equatorial bonds become axial, and axial become equatorial.
Atropisomerism: Atropisomerism is stereochemistry arising from the restricted bond rotation that creates a chiral axis. Atropisomers are stereoisomers resulting from hindered rotation about one or more single bonds between two planar moieties where the energy barrier to rotation is high enough to allow for the isolation of individual conformers. The conformers are detectable by NMR if half-lives of conformers exceed 10–2 sec. and can be isolated if their half-lives are above 1000 sec.
The name atropisomerism (from Greek, a = not and tropos = turn) was introduced by Kuhn in 1933 but it was first detected in 6,6-dinitro -2, 2’-diphenic acid by Christie in 1922. The bulkier groups on the ortho position of the biphenyl ring restrict the rotation through the C–C bond giving two enantiomers and resolvable at room temperature.
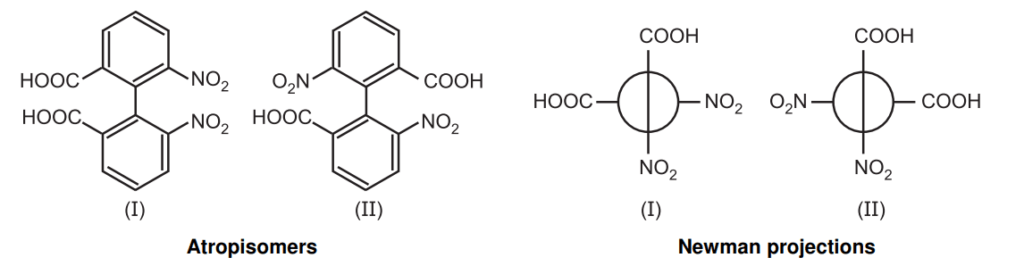
Atropisomerism induces time-dependent inversion of chirality via bond rotation generating atropisomers having different pharmacokinetic, biological, and toxicological profiles.
Nomenclature: The Cahn-Ingold-Prelog system suggested assigning stereochemical descriptors (R, S descriptors) to molecules with axial chirality. All four groups are ranked with overall priority given to the groups on the front atom of the Newman projection. The two configurations are termed Ra and Sa in analogy to the conventional R/S.
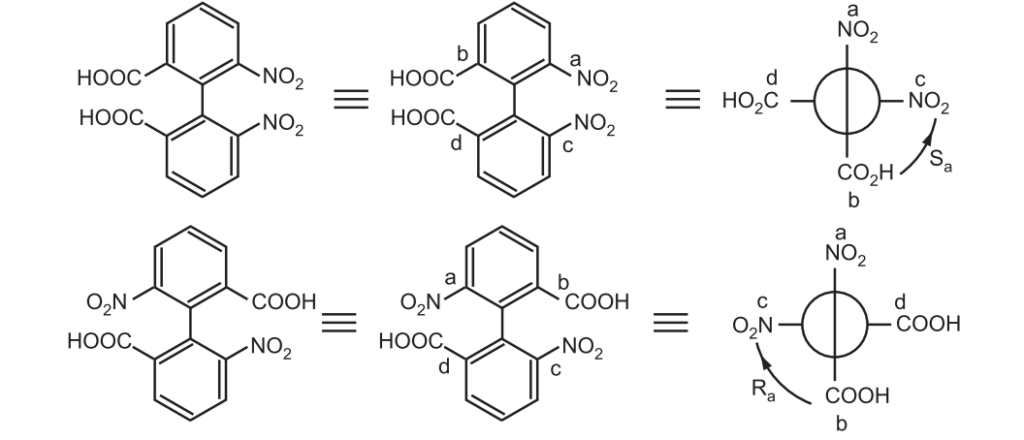
In yet another nomenclature, if the sequence a-b-c-d of the substituents is clockwise, the configuration is called P or ∆. If it is counter-clockwise, the configuration is called M or ^.
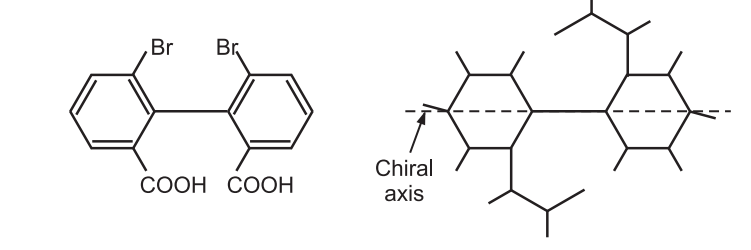
Atropisomerism is also called as axial chirality. The chirality is not simply a center or a plane but an axis. The simple symmetric biphenyl is achiral. Only biphenyl having different substituents at the ortho position contains a chiral axis. The biphenyl rings turn perpendicular to each other in order to minimize steric clashes the biphenyl bond is restricted. For example,
Make sure you also check our other amazing Article on : Synthetic Evidence of Benzene