Aim: To determine the radiation constant of Iron.
Requirements:
Apparatus/ Instruments: Iron Cylinder With Hole Or Cavity, Thermometer, Glass tripod Stand/ Wooden Slab, Iron Tripod Stand, Bunsen Burner, Gas Cylinder, Match Box Or Gas Lighter, Weighing Balance, Screw Gauge And Tongs, etc.
Principle:
Heat transfer is a major unit operation in pharmacy. Heat flows from a region of high temperature to a region of low temperature. Heat may flow by one or more of three mechanisms viz. Conduction, convection, and radiation.
Heat is lost from the hot metal cylinder surface to the surrounding atmosphere by means of conduction and radiation. Heat transfer by radiation occurs, and energy transfer through space by means of electromagnetic radiation (waves). Thus a body acts as an emitter, and then energy is transmitted through the intervening spaces, and it is effective even in a perfect vacuum or inter-spacious space. The amount of thermal energy radiated by a surface is increased rapidly with increasing temperature when heat flows by actual mixing of warmer portions with cooler portions of the same material. This mechanism is known as Convection.
A freely suspended body loses heat by conduction, convection, and radiation until it reaches room temperature. This is an equilibrium condition. The present experiment demonstrates the pattern of cooling of a hot body by combined convection and radiation. In this system, the heat loss through convection is neglected, since the movement of the molecule is negligible. As the metal cylinder is freely suspended without any contact with the metal the heat loss through conduction is minimum. For this reason, the metal cylinder is placed on a glass tripod stand. Thus heat loss by radiation is highlighted. In conduction, the energy transfer occurs by the transmission of momentum of individual molecules.
The total amount of radiation emitted by a black body may be calculated using Stephan Boltzman’s law:
q= bAT 4
where,
q = Energy radiated per second, W (or J/s)
A=Area of the radiating surface, m 2
T =Absolute temperature of the radiating surface, K
b = Constant, W/m 2 .K
According to the above equation, the rate of heating depends upon the temperature and surface area of the emitter. At the same time, it also depends upon the absorption capacity of the material to be heated.
The difference in the temperature of a hot body and the ambient is the temperature gradient for the heat loss by radiation. The radiation constant is calculated using the following equation:
Where,
M= weight of cylinder (g)
S = specific heat of metal (iron), J/kg.K
= temperature gradient (rate of change of temperature, K/sec)
T1 = temperature of the metal, K
T2 = room temperature, K
β = convection constant = 2.8
A = surface area of the cylinder in m 2
α = radiation constant (W/m 2.K 4)
Procedure:
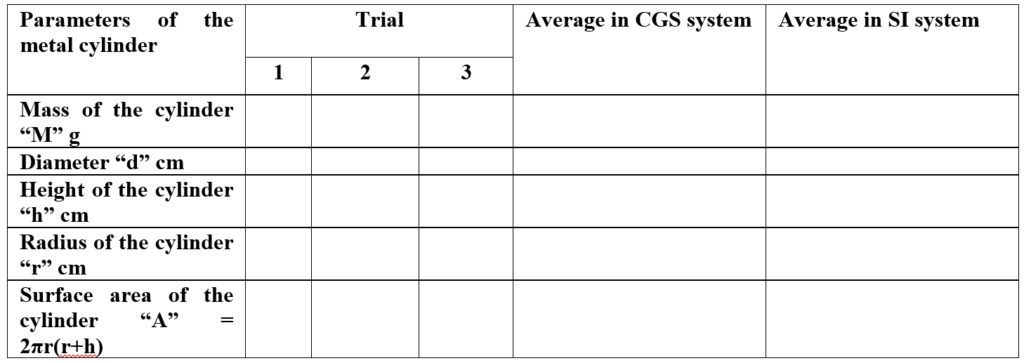
- A metal (iron cylinder) whose surface is smooth is cleaned and weighed on a weighing balance and the average weight is recorded in the observation table.
- The diameter and height of the cylinder are measured and the radius of the iron cylinder is calculated and recorded in the observation table.
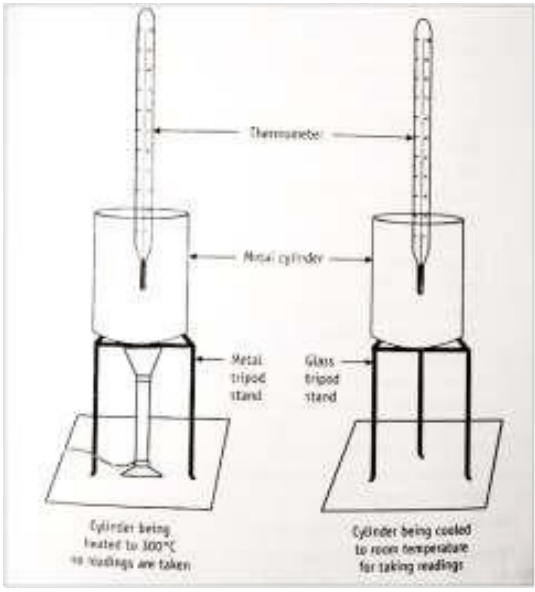
- The thermometer is placed into the cavity of the iron cylinder and fixed to a stand using a thread (A simple 360 o C thermometer can be used).
- The iron cylinder is placed on a tripod stand and heated with the help of a Bunsen burner or with a heating device to above 300 o C.
- Ager reaching a constant maximum temperature, the metal cylinder is readily transferred to the glass tripod stand /wooden slab using a long tong without touching any surface.
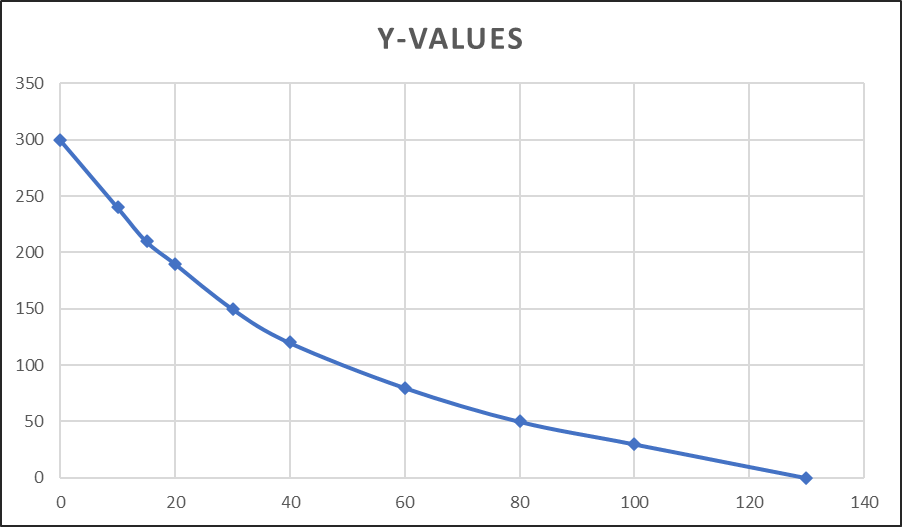
- Slowly the temperature of the hot body decreases. The decrease in temperature is noted every 5 minutes interval and data is recorded in the observation table.
- A graph is plotted by taking time (minutes) on x- the axis and temperature on the y-axis.
- Depending on the temperature at which the radiation constant is to be determined a tangent dQ/dt is drawn at that temperature. The slope of the tangent is calculated, which represents the rate of loss of heat by the hot body.
- From the collected data the radiation constants are determined at a specific temperature.
Observations:
Parameters of metal cylinder:
Specific heat of iron “S” = 106 (at 25 o C in J/kg.K)
Convection Constant “β” = 2.8
Room temperature, T2 = _________K
Desired temperature T1 = _________K
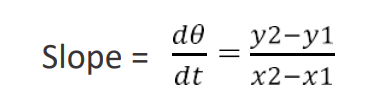
The calculation for determination of “α” at particular temperatures:
Use the following tables for tabulating the results:
| Time (minutes) | Temperature (o C) | Time (minutes) | Temperature (o C) | Time (minutes) | Temperature ( o C) |
The radiation constant is calculated using the following equation:
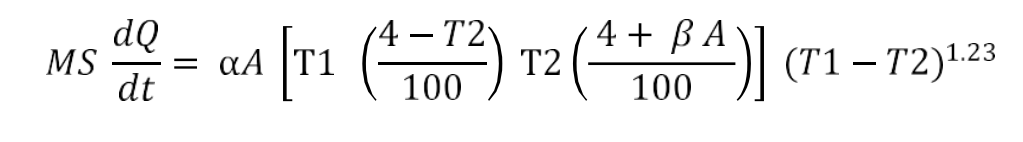
RESULT:
The radiation constant “α” of Iron is =
Make sure you also check our other amazing Article on: How do you calculate the heat transfer coefficient of water?