Aim: Determination of radiation constant of glass (unpainted and painted)
Requirements:
Apparatus/ Instruments: Round bottom flask along with neck (500ml), glass tripod stands, digital thermometer, tong, painted (white green colored) round bottom flasks with a long neck.
Principle:
Heat transfer by radiation involves the transfer of energy in the form of electromagnetic waves. These become significant at higher temperatures. All solid bodies radiate energy when their temperatures are above absolute zero. The radiant energy emitted by a hot body is expressed by Stefan Boltzmann’s law which states the rate of radiation emitted by a body.
Q= bAT 4
Where,
Q = Energy emitted per second W (or J/s)
A=Area of the radiating surface, m 2
b = absolute temperature of the radiating surface, K
T= constant W/m 2. k 4
According to the above equation, the rate of heating depends on the temperature and surface area of the emitter. At the same time, it also depends upon the absorption capacity of the material to be heated. The difference in the temperature of a hot body and the ambient is the temperature gradient for the heat loss by radiation.
Radiation constant α is calculated using the following equation:
Total heat lost by the body = heat loss due to radiation + heat loss due to convection
OR
Radiation constant α is calculated using the following equation:
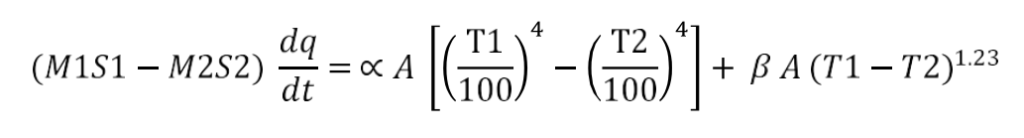
Where,
M1=mass of water W(Kg)
M2=mass of the round bottom flask (unpainted / painted), kg
S1 = specific heat of water, J/Kg. K
S2= specific heat of glass, J/Kg.K
dQ/dt= rate of change of temperature (rate of heat loss by the apparatus) (K/sec)
T1= Temperature of the metal body, K
T2 = Temperature of ambient (room temperature) K
β = convection factor
A= surface area of the heat transfer m 2
α = radiation constant(W/m2 . K 4 )
All the values are determined experimentally and substituted in the above equation to determine α (radiation constant)
The special feature of heat transmission by radiation is that radiant energy penetrates a certain distance in the material. Beyond a particular point, heat transfer by radiation becomes ineffective. For this reason, the formation of surface skin must be avoided. When glass apparatus is painted with silver oxide, the rate of radiation decreases because painting acts as insulation from radiation. Heat transfer is a major unit operation. Heat flows from a region of high temperature to a region of low temperature. Understanding heat transfer requires the study of the mechanism & rate of the process. Heat may flow by one or more of 3 basic mechanisms.
- Conduction: itis a process in which heat flow in a body is achieved by the transfer of the momentum of the individual atoms or molecules without mixing. Example: Flow of heat through the metal shell of a boilert6akes place by conduction as far as solid shell or wall is considered. This mechanism is limited to solids & fluids.
- Convection: It is a process in which heat flow in a body is achieved by the actual mixing of a warmer portion with the cooler portion of the same materials. Example: Heating by water on a hot surface (coil-type water heater) is mainly convection. Convection currents are responsible for winds, land/sea breezes, ocean currents, etc.
- Radiation: It is the process in which heat flows through spaces by means of electromagnetic waves. Example: A black surface absorbs most of the radiation received by it, it will transfer such absorbed energy quantitatively into heat.
Thermal radiation:
Heat transfer by radiation is known as thermal radiation. All solid bodies radiate energy when their temperature is above absolute zero.
Application:
Radiation energy is used in different processes, where heat is necessary.
For example, drying solids is attempted using microwave radiation, IR radiation, etc.
Procedure:
- Around the bottom flask (unpainted)is cleaned & dried.
- The weight of the flask is determined (M2, kg)& reporting
- The diameter(D)of the round bottom flask is determined & reported.
- The diameter(d) of the neck of the flask is determined & reported.
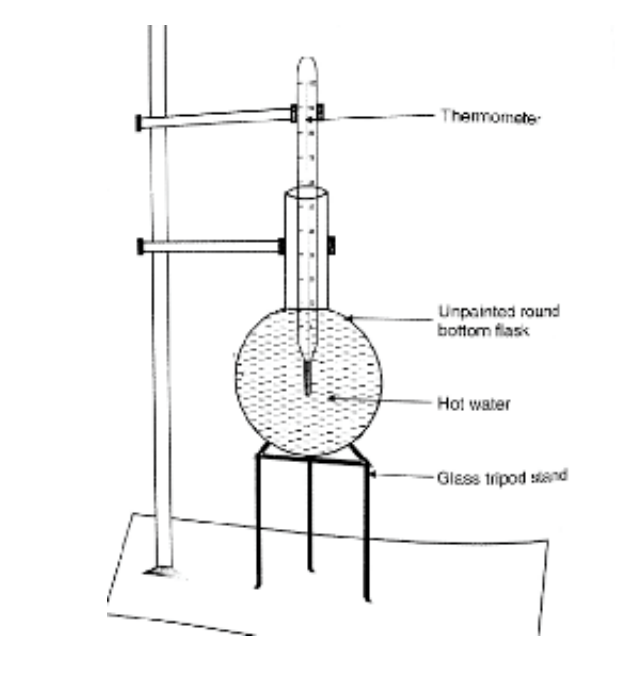
- Boiled hot water is prepared & separately measured volume of hot water is transferred (M1). The external surface of the round bottom flask is thoroughly dried & cleaned. The flask with hot water is placed on the tripod stand.
- The thermometer (110 0 C) is dipped to the centre of the flask &tied at the top to an iron stand.
- Slowly the temperature of the hot body (water) decreases. The decrease in temperature is noted every minute. The data are recorded.
- A graph is plotted by taking time on the X-axis & temperature on the Y-axis. Normally a curve is obtained.
- Depending on the temperature at which the radiation constant is determined, a tangent is drawn at that temperature. The slope is calculated. This parameter is related to the rate of heat loss(dq/dt).
- The radiation constant is determined at that temperature.
- The same procedure is repeated with a round bottom flask painted with silver oxide white color or green color.
Precaution:
- The glass surface should not be touched with and s white taking readings
- Care should be taken while transferring ring hot water quantitatively into the round bottom flask
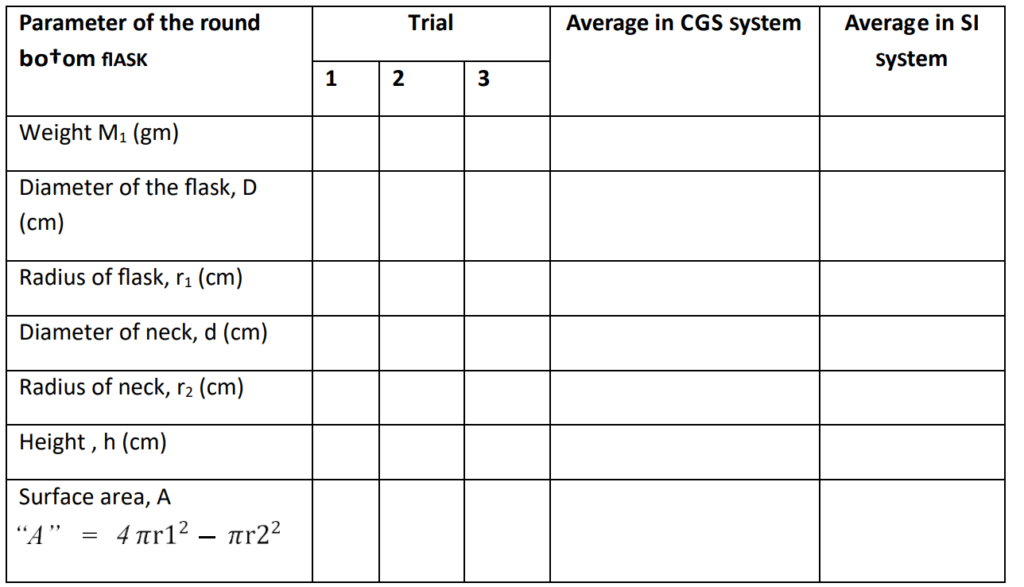
Observation
Table Parameter of the glass apparatus for heat transfer
Volume of hot water transferred, M1 =ml or _________gm _____kg
Heat loss by convection, β = 2.8
Specific heat of water, S1= 4190 J/kg. K
Specific heat of glass, S2= 500 J/kg. K
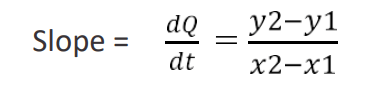
The calculation for determination of “α” at particular temperatures:
Use the following tables for tabulating the results:
| Time (minutes) | Temperature (o C) | Time (minutes) | Temperature (o C) | Time (minutes) | Temperature ( o C) |
The radiation constant is calculated using the following equation:
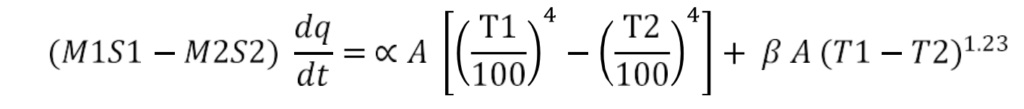
Result:
The radiation constant of glass (painted and unpainted) is =
Make sure you also check our other amazing Article on: How do you find the radiation constant?