Aim: To determine the specific surface area of Talc by adsorption method.
Principle of Specific Surface Area
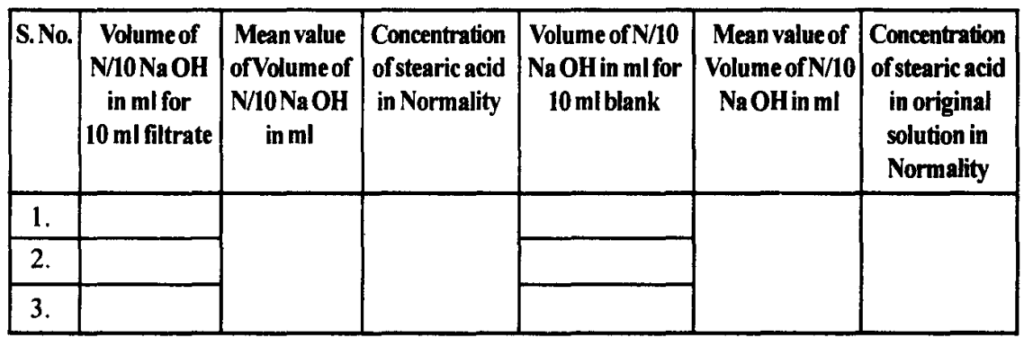
The specific surface area is defined as the surface area for unit weight or per unit volume. When a dissolved solid is adsorbed onto an adsorbent as a monolayer, the surface area of the solid is equal to the surface area of the adsorbent.
If the surface area of the adsorbed solid is determined, then the surface area of the adsorbent and specific surface area can be calculated. However, the surface area of one molecule of adsorbed solid must be known.
Stearic acid is a linear molecule that gets adsorbed onto an adsorbent as a monolayer. The stearic acid adsorption on talc can be used in this experiment to find out the specific area of talc.
Materials and apparatus required: Talc, stearic acid, conical flasks, burettes, pipettes, N/10 NaOH, and phenolphthalein.
Procedure:
- 100 ml of 1.0% stearic acid solution is prepared in neutralized methanol (methanol is slightly acidic and is neutralized with dilute alkali).
- Around 5 g of talc is accurately weighed and taken in a stoppered conical flask.
- 50 ml of 1.0% stearic acid in neutralized methanol is added to the conical flask containing talc.
- The mixture is thorough!)’ shaken from time to time for about 1 hr (The time required to attain adsorption equilibrium). The mixture is then left undisturbed for around 5 minutes.
- The mixture is filtered through dry filters into a dry container (conical flask).
- 10 ml of the filtrate (containing unadsorbed stearic acid) is pipetted out with a bulb pipette and titrated against N/10 NaOH solution using phenolphthalein as an indicator.
- A blank titration is also performed using the original 10 ml of 1.0 % stearic acid solution.
Observation and Calculation:
Room Temperature: _______0C
Weight of talc taken = ______g (Let it be m g).
Titration with N/10 NaOH.
If the Normality of original stearic acid solution = N1 and Normality of filtrate (Equilibrium Normality) = N2,
Then (N1 – N2) equivalent / liter stearic acid is adsorbed.
As only 50 ml of stearic acid solution was used, the quantity of stearic acid adsorbed
\frac{(N_1-N_2)\times Equivalentweightofstearicacid\times50}{100}g(Let\;it\;be\;x\;g)
(Equivalent weight of stearic acid = Mol. wt of stearic acid = 284)
Now, m g of talc adsorbed x g stearic acid.
The surface area of m g talc = Surface area of x g stearic acid.
No.\;moleculerinxgofstericacid=\frac{6.023\times10^{23}}{284}\times\;x
[As 1 g molecule of any substance contains 6.023 x 1023 molecules; Avogadro’s No.]
1 molecule of stearic acid has an area of 20.46 x 10-16 sq. cm.
Thus, \frac{6.023\times10^{23}}{284}\times\;x of stearic acid will have an area of \frac{20.46\times10^{-16}\times6.023\times10^{23}}{284}\times\;x\;sq.\;cm
Therefore, The surface area of 1 g talc would be \frac{20.46\times10^{-16}\times6.023\times10^{23}}{284}\times\;x\;sq.\;cm
[The quantity of stearic acid adsorbed can simply be calculated from the relation:
1 ml of N/10 NaOH = 0.0284 g of stearic acid.
Quantity of stearic acid adsorbed per 10 ml of the mixture = (Vol. of N/10 NaOH for blank – Vol. of N/10 NaOH for filtrate) x 0.0284 g]
Report: The specific surface area of talc is ____ sq. cm. /g.
Make sure you also check our other amazing Article on: How does Andreasen’s pipette method work?