Aim: To determine the average particle size and study distribution pattern, using Andreasen Pipette, of the supplied powder sample (Zinc Oxide).
Principle: Particle size determination and distribution study using Andreasen Pipette utilizes the principle of sedimentation (stokes’ law).
Stokes’ law:
Rate\;of\;settling=\frac ht=\frac{(d^2(\rho_s-\rho_1)g)}{18\eta}
Where,
d = diameter of the particle,
\rho_s= density of solid which is dispersed in the liquid
\rho_1= density of the liquid in which the solid is dispersed,
g = acceleration due to gravity,
\eta= viscosity of the medium,
h = height of falling,
t = time of falling.
The diameter can be calculated by rearranging stokes’ equation:
d=\sqrt{\frac{18\eta}{(\rho_s-\rho_1)gt}}
Andreasen pipette is a vessel, as shown in the figure, consisting of a 550 ml vessel containing a 20 ml pipette sealed into a ground glass stopper. The lower end of the pipette is 20 cm below the surface of the suspension from where sampling is done.
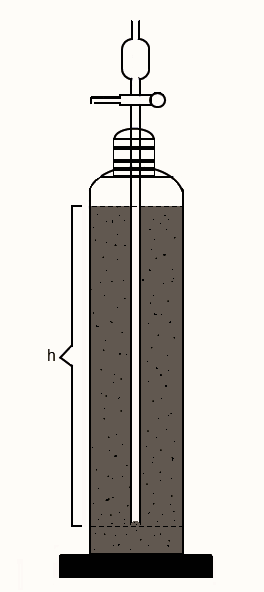
After the addition of dispersion to the apparatus, the withdrawal of samples at different intervals quantifies the number of solids settled at that time interval. The number of powders present in each withdrawn sample can be determined through a suitable assay method. Gravimetry is preferred sometimes, as it does not involve any reagent. The weight of solid residue in each sample withdrawn is the weight fraction having particles of size less than the size obtained by the stokes’ law calculation for that time period of settling. The weight of each sample residue is known as weight undersize and the sum of successive weights is cumulative weight under size.
Materials and apparatus required: Andreasen pipette, weighing balance, sample (zinc oxide), liquid medium (water), deflocculating agent (sodium citrate)
Method:
- A suspension of 5.5 g zinc oxide in 50 ml water is prepared to contain 2.75 g sodium citrate as the deflocculating agent. The suspension is diluted to 550 ml with sufficient water to give a final concentration of 1%.
- The suspension is introduced into the vessel up to the 550 ml mark.
- The vessel is stoppered and shaken to distribute the particles uniformly throughout the suspension.
- The apparatus fitted with the pipette in place is clamped securely, preferably in a constant temperature bath.
- 20 ml samples are withdrawn at a predetermined time interval and discharged · by means of a two-way stopcock.
- Samples are evaporated and weighed (correction for the quantity of flocculating agent is required).
Observation and Calculation:
The particle diameter corresponding to the various time periods is calculated from stokes’ law: The data are:
Density of zinc oxide = 5.60 g/cc,
Acceleration due to gravity = g =981 cm/s2
The density of water = in g/cc (value can be determined or obtained referring to the appendix at room temperature; however, for the practical purpose may be considered as 1 g/cc),
The viscosity of water = in g cm-1.s-1 (value can be determined or obtained referring to the appendix at room temperature, however, for the practical purpose may be considered as 1 cp i.e., 0.01 g cm-1.s-1),
Height = h = 20 cm.
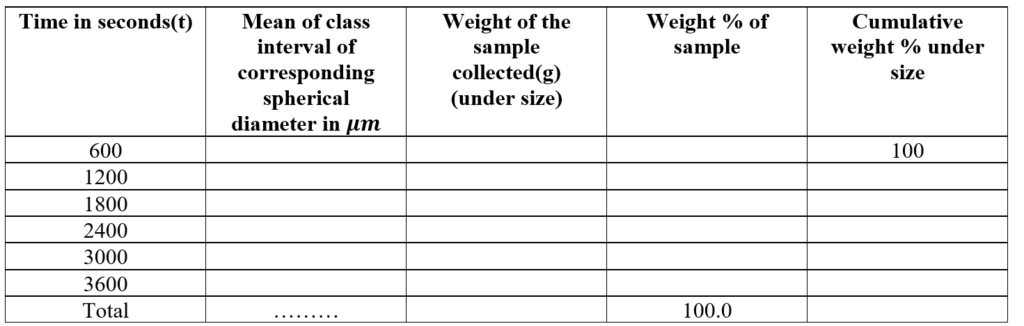
The particle size distribution can be expressed as the plot of particle diameter on the X-axis and the cumulative weight percent undersize on the Y-axis.
The geometric mean diameter (the particle size at a 50% probability level) can be calculated from the curve.
Report: The average particle size (particle size at 50% probability level) is _______\mu m The distribution pattern is shown in the figure as cumulative under size.
Make sure you also check our other amazing Article on: Determination of particle size distribution by sieving method