There are various techniques of pharmaceutical analysis which can be divided into two major categories.
- Qualitative analysis
- Quantitative analysis
1. Qualitative Analysis:
This category of analysis involves various test procedures that are designed for the identification of compounds in the sample. These tens results confirm the presence or absence of a compound in the sample to be analyzed.
Examples: Colour reaction tests, limit tests, melting point and boiling point determination for identification, precipitate formation, etc.
2. Quantitative Analysis:
This category of analysis involves the quantitative determination of compounds in the sample. Generally, the quantitation has been done on the basis of some physical properties of components. Quantitative analytical techniques are further classified as follows:
What are the different techniques of pharmaceutical analysis?
Techniques In Pharmaceutical Analysis
1. Chemical Methods
(a) Volumetric, (b) Gravimetric, (c) Gasometric
2. Physico-chemical Methods or Instrumental Methods
3. Microbiological Methods
4. Biological Methods
Chemical Methods
(a) Volumetric Methods: In volumetric methods, the measurement of the volume of solution is taken as a parameter for assay. The volume of known strength of a solution that is required to react completely with the substance to be analyzed is measured. The quantity of analyte is determined from the volume of the solution by calculation. The solution or reagent is called a titrant and the analyte to be analyzed is termed a titrate. The ideal characteristics of this method to be considered are that, the reagents selected should react rapidly with the analyte, the reaction must be complete and there should be some method of detection to detect the end of the reaction. Volumetric or titrimetric methods are rapid and user-friendly compared to gravimetric methods, thus preferred. Volumetric methods are classified into different types depending upon the type of reactions involved in the reaction which are as follows:
- Neutralization titrations
- Precipitation titrations
- Complex metric titrations.
- Non-aqueous titrations
- Oxidation-reduction titrations
(b) Gravimetric Methods: In Gravimetric analysis, quantitation is done on the basis of the weight of the compound. This process involves isolation and weighing of the compound of known composition, ie. purest form. The analysis is carried out by various processes such as precipitation, volatilization, electro-analytical, etc. Precipitation and volatilization are widely employed methods. Gravimetric analysis is time-consuming compared to other techniques. It is useful for the examination of compounds for the presence of impurities.
(c) Gasometrical Methods: In this method, the measurement of the volume of gases forms the basics of analysis. When a chemical reaction is carried out under a specific process, the volume of gas evolved or absorbed in the reaction is measured. The measured volume is corrected to standard conditions of temperature and pressure. Gas burettes or nitro-meters are used for the measurement of the volume of gas. For example, gases that are measured by gasometrical methods are cyclopropane, carbon dioxide, nitrous oxide, oxygen, octal nitrate, nitrogen, amyl nitrate, ethylene, and helium.
Instrumental Methods
These methods involve the usage of instruments to measure the physical or physiochemical properties of the compound to be analyzed thus leading to the quantitation of the compound. Depending on the physical property of the compound various instruments have been used for the measurement. The measurement of change in properties such as electric current, potential, electrical conductivity, optical density, scattering of radiation, absorption, emission, refractive index, etc. using suitable, reliable, and sensitive instruments. Some of the physical properties and corresponding equipment used are listed as follows.
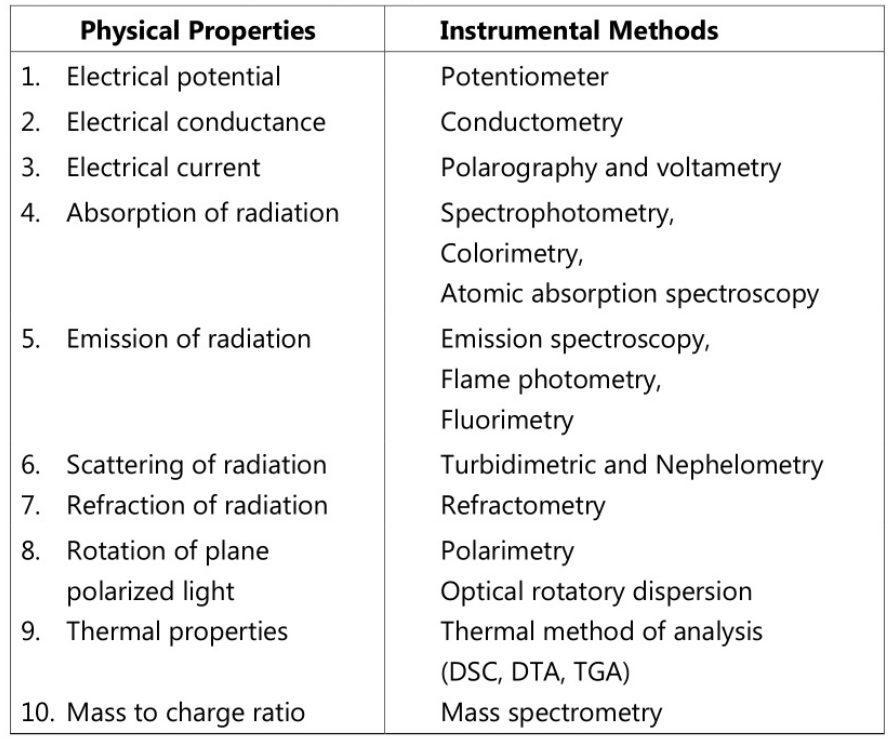
Further, instrumental methods involve another major area of analysis for separation purposes called chromatography. Chromatographic techniques are the separation techniques used to separate components from a mixture of two or more compounds. They use generally a stationary phase and a mobile phase for separation using adsorption and partition as the major principles. Various techniques are involved in chromatography including column chromatography, HPLC, thin layer chromatography, HTLC, paper chromatography, gel chromatography, gas chromatography, flash chromatography, ion exchange chromatography, and ion pair chromatography. These techniques have extensive applications in the pharma industry.
Microbiological Methods
Microbiological methods are employed for compounds, especially for antibiotics, for which chemical methods are not useful. This method involves the determination of inhibition of the growth of bacteria by the substances to be analyzed in comparison with the standard compound. On the basis of the result, the therapeutic efficacy of the antibiotics is decided. The methods which are generally used include the cylinder plate (or cup plate) method and the Turbidimetric (or tube assay) method.
Biological Methods
Biological assays are carried out to observe the biological effect of the drug on some type of living matter. They are also called bioassays. These are recommended when the chemical or physical methods are not capable to estimate the potency of a drug. These methods are carried out by comparing the biological effect of the sample to be tested with the biological effect produced by a standard compound in identical test conditions. However, the results of biological assays vary due to the difficulties in maintaining identical conditions thus the errors are also to be considered and corrected. Bioassays involve the measurement of various parameters including the weight of tissues of organs, the weight of organs, blood parameters such as blood glucose, cholesterol, urea, enzyme concentration, etc. In some assays, even the physiological symptoms also have been considered.
Make sure you also check our other amazing Article on: Definition And Scope of Pharmaceutical Analysis
