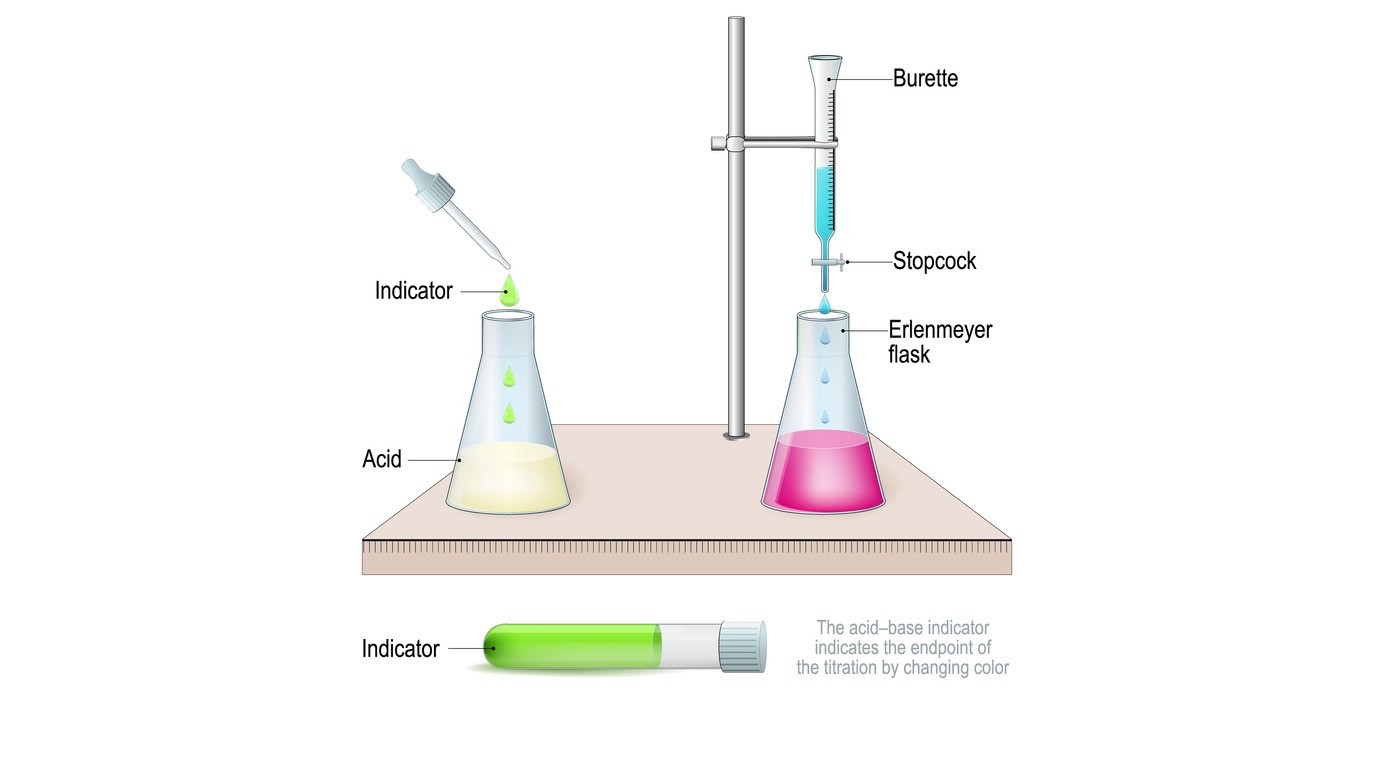
Aim: To determine the solubility of Oxalic Acid in water (titrimetric analysis method of estimation).
Principle titrimetric method
The concentration of a solute in a solution that is in equilibrium with the solute is how solubility is expressed mathematically (solid). This implies that a solute’s solubility is equal to its concentration in a saturated solution.
The solubility of solids in solvents is often defined in relation to the solute and solvent that produce a saturated solution, even though concentration can be expressed in a variety of units. The number of milliliters of solvents that 1 gram of a solute will dissolve in to create a saturated solution is how solubility is expressed in the pharmacopeia.
The titrimetric analysis method of determining solubility is to find the amount of solute in a saturated solution, calculate the amount of solvent in volume, then calculate the amount of solute in weight to represent solubility.
In this case, the concentration of oxalic acid in the saturated solution is estimated by the titrimetric method using a standard sodium hydroxide (NaOH) solution.
Apparatus and Materials required: Conical flask, pipettes, burette, std. sodium hydroxide solution, oxalic acid, phenolphthalein, and water.
Process:
- An increasing amount of oxalic acid is added to about 50 ml water in a conical flask with shaking until the solution is saturated and a part of the solid is left undissolved (around 08 grams of oxalic acid is required).
- The solution is filtered and 10 ml of the filtrate is pipetted out into a conical flask and titrated against standard Nil 0 sodium hydroxide solution. Three observations are taken.
- The concentration of oxalic acid is determined by the titer value.
- The density of the filtrate is determined using a pycnometer.
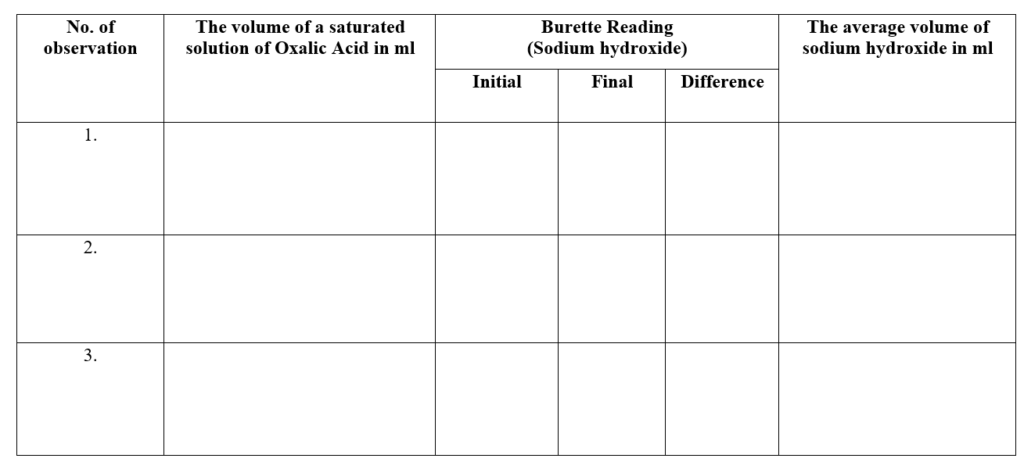
Observation:
Room Temperature: ___________ °C
Titration of oxalic acid solution with std. sodium hydroxide
Calculation:
Amount of oxalic acid present in 10 ml of the saturated solution
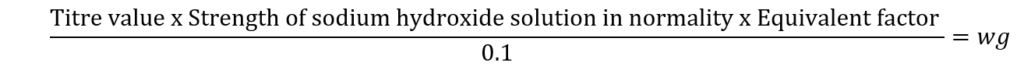
The equivalent factor is 0.0053 (1 ml of Nil 0 sodium hydroxide solution is equivalent to 0.0053 g of oxalic acid)
The density of saturated solution = x g/ml.
The density of water at room temperature can be referred from the appendix (for rough calculation density of water may be considered as 1 g/ml).
The volume of solvent (water) present in 10 ml saturated solution
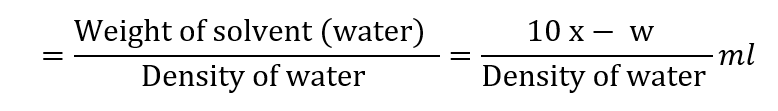
Therefore, Solubility in terms of the number of ml of solvent required for 1 part of the solute:
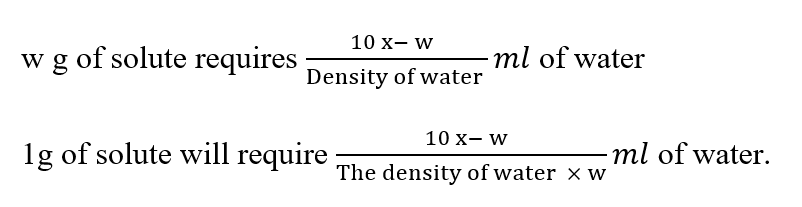
Report: The solubility of oxalic acid in water at room temperature (specify) is 1 ml.
(Note: The solubility of oxalic acid is 1 g in 7 ml water)
Make sure you also check our other amazing Article on: Determination of Solubility By Gravimetric Method