Aim: To determine the distribution coefficient of iodine between carbon tetrachloride and water.
Principle: When a dissolved solid is distributed between two immiscible liquids/solvents, the ratio in which it distributes is called the distribution coefficient or partition coefficient. If the solute is in the same molecular condition in both solvents, the distribution coefficient is constant and is dependent on temperature. This is also known as the partition coefficient.
When a substance is distributed between two immiscible solvents: A and B,
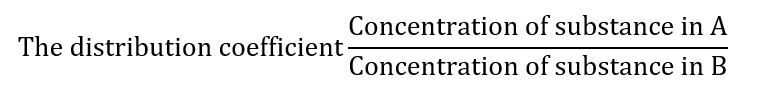
In pharmaceutical sciences, the organic solvent and water system are important.
The distribution or partition coefficient can be expressed as between the organic phase and water, or between water and organic phase.
Though the concentration of the substance in the organic and water phase needs to be mentioned in molar concentration; as the distribution coefficient is a ratio term, the units get nullified. The concentration can thus be expressed in any unit if the solute is in the same molecular state in both phases.
The molecular state of iodine in both the solvents: carbon tetrachloride and water are the same.
Apparatus and Materials required: Stoppered bottles, carbon tetrachloride, water, iodine, std. Nil 0 sodium thiosulphate, std. Nil 00 Sodium thiosulphate solution, potassium iodide, starch, pipettes, and burettes.
Process:
1. Distribution of Iodine in two phases:
- A nearly saturated solution of iodine in 150 ml carbon tetrachloride is prepared in a beaker by adding a few crystals of iodine to carbon tetrachloride and stirring with a glass rod.
- The following mixtures of iodine solution in carbon tetrachloride and water are prepared in three glass-stoppered bottles:
| Bottle number | Volume of pure carbon tetrachloride, ml | The volume of iodine solution in carbon tetrachloride, ml | The volume of water, ml |
- After tightening the stopper, the bottles are shaken vigorously for a few hours. At frequent intervals of 1 to 2 minutes, the stoppers are removed to release pressure.
- The bottles are then kept undisturbed allowing the liquids to separate into two layers. The lower layer is carbon tetrachloride and the upper one is that of water. (Note: The separating funnels can also be used in place of glass stoppered bottles.)
2. The determination of the concentration of Iodine in two phases:
- 50 ml of the aqueous layer is pipetted out from the first bottle into a conical flask containing about 5ml 10% potassium iodide solution and 1 ml starch solution (taking care that the tip of the pipette is above the interface in order to prevent any carbon tetrachloride getting into the pipette). It is then titrated against Nil 00 sodium thiosulphate solution. Similarly, 50 ml quantities of the aqueous layer are withdrawn from other bottles and titrated.
- 5 ml of carbon tetrachloride layer is pipetted out into a conical flask containing 20 ml of 10% potassium iodide solution and I ml starch solution as indicator. It is then titrated against std. Nil 0 sodium thiosulphate.
(While titrating the organic layer (carbon tetrachloride), the titration f1ask must be shaken continuously; otherwise, the endpoint may cross without the appearance of the purple color of the non-aqueous layer.)
Similarly, 5 ml of carbon tetrachloride layer is withdrawn from other bottles and titrated.
| Bottle number | The volume of N/100 sodium thiosulphate for 50 ml aqueous layer (ml) | The concentration of iodine in the aqueous layer (molarity) | The volume of N/100 sodium thiosulphate for 5 ml tetrachloride layer (ml) | The concentration of iodine in the carbon tetrachloride layer (molarity) | Distribution coefficient | Average valve |
Observation and Calculation :
Room temperature: ____ 0C.
(1 ml of N/10 sodium thiosulphate is equivalent to 0.5 x 10-4 g mole Iodine.)
Report: The distribution coefficient of iodine between carbon tetrachloride and water is at °C.
Make sure you also check our other amazing Article on: How is titrimetric analysis calculated?
