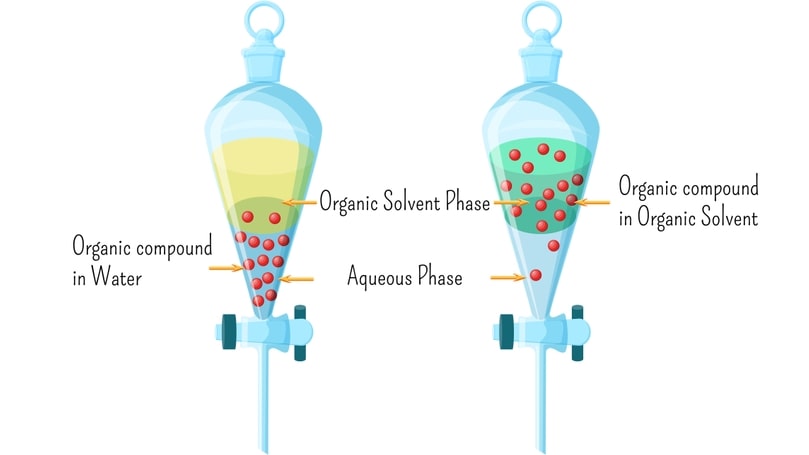
Distribution Law In pharmaceutical practice, often a single substance is dissolved in two immiscible phases’ i.e. two liquid phases in contacts that do not mix, such as chloroform and water. When an excess amount of solute is added to two immiscible liquid phases, it distributes itself between these phases until saturation, if mixed by shaking vigorously. If an insufficient amount of solute is added it distributes in a definite ratio. The term partition coefficient is commonly referred to as the equilibrium distribution of a single substance between two solvent phases separated by a boundary. If a third substance dissolves to some extent in both phases, the partition coefficient is the ratio of the amounts of the third substance dissolved in two phases. Partition coefficients are sometimes called distribution coefficients. The partition coefficient is a measure of drugs lipophilicity and is an indication of its ability to cross cell membranes. It is commonly determined using an oil phase of octanol or chloroform and water.
If there is possible confusion with the extraction factor or mass distribution ratio the term concentration distribution ratio should be used. The terms distribution coefficient, extraction coefficient, and, wherever appropriate, scrubbing coefficient, stripping coefficient are widely used as alternatives but are not recommended. If they must be used in a given situation the term ratio is preferable to coefficient. In equations relating to aqueous/organic systems, the organic phase concentration is, by convention, the numerator and the aqueous phase concentration the denominator. In the case of stripping ratio, the opposite convention is sometimes used but should then be clearly specified. In the past, there has been much confusion between the distribution ratio as defined above, the value of which varies with experimental conditions, for example, pH, presence of complexing agents, the extent of achievement of equilibrium, etc. and the true partition coefficient which is by definition invariable or the partition coefficient or distribution constant which applies to a chemical species under specified conditions. For this reason, the terms distribution constant, partition constant, partition coefficient, partition ratio, and extraction constant should not be used in this context. The use of the ratio of light phase concentration to heavy phase concentration is ambiguous and is not recommended. The distribution ratio is an experimental parameter and its value does not necessarily imply that distribution equilibrium between the phases has been achieved.
Thermodynamic Deduction of Distribution Law
The chemical potential of solute is at equilibrium in both aqueous and organic immiscible solvents. The chemical potential (µw) of solute in the aqueous phase is expressed as:
µw = µw°+ RT ln Cw……………(1)
where, µw°standard chemical potential of solute in the aqueous phase, Cw is the concentration of solute in the aqueous phase, R is gas constant and T is the absolute temperature. Similarly, the chemical potential of solute in the organic phase is expressed as
µorg = µorg°+ RT ln Corg……….(2)
where Corg is the concentration of solute in the organic phase and µorg° is the standard chemical potential of solute in the organic phase. At the equilibrium upon distribution:
µw = µorg……………………………..(3)
µw°+ RT ln Cw = µorg°+ RT ln Corg
RT ln (Cw/Corg) = µw − µorg…………(4)
At a given temperature, the standard chemical potentials of solute in aqueous and organic phases are constant.
RT ln (Cw/Corg) = Constant………(5)
Therefore, the ratio Cw/Corg is constant at a given temperature. The ratio constant is called as distribution coefficient or partition coefficient and it depends upon the amount of solute added. Equation (5) represents the Nernst distribution law.
The ability of a drug to dissolve in a lipid phase when an aqueous phase is also present often referred to as lipophilicity can be best characterized by a partition coefficient. The true or intrinsic partition coefficient can be defined as the ratio of the unionized drug distributed between the organic and aqueous phases at equilibrium. It is expressed for unionizable molecules as
Ko/w = Co/Cw…………….(6)
where Co is a concentration of unionized drugs in the organic phase and Cw concentration of unionized drugs in the aqueous phase. For ionizable molecules (acids, bases, salts) it is expressed as
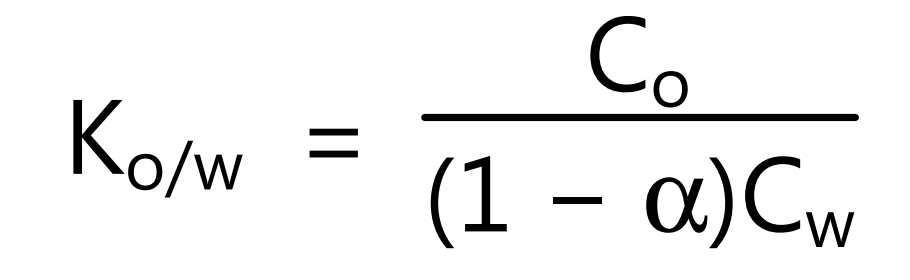
In equation (7) the term α is the degree of ionization in an aqueous solution. Since partition coefficients are difficult to measure in living systems, they are usually determined in vitro using n-octanol as the lipid phase and a phosphate buffer of pH 7.4 as the aqueous phase. This permits standardized measurements of partition coefficients. The Ko/w is expressed in the form of log Ko/w as the measure of lipophilicity. If added solute has an equal molecular weight in both phases, the ratio of the concentration of solute in both phases is found to be constant. These concentrations of solute in aqueous and organic phases are expressed in g/liter or gram equivalent/liter. Being the ratio of two concentrations the constant, partition coefficient is dimensionless value. The value of Ko/w is unitless. It is necessary to specify in which of these two ways the partition coefficient is being expressed. No convention has been established with regard to whether the concentration in the aqueous phase or in the organic phase should be placed in the numerator. Therefore, the partition coefficient may be expressed as
Ko/w = Cw/Co
Or,
K = Co/Cw………………(8)
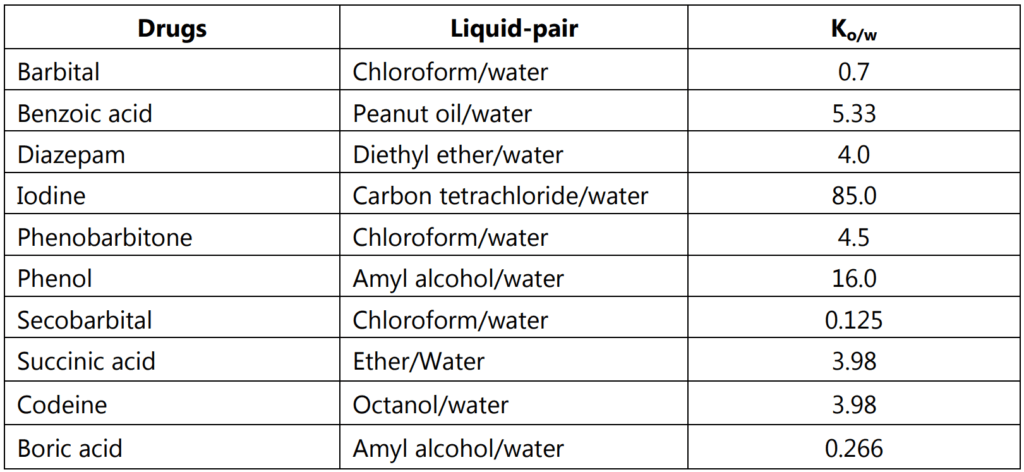
The partition coefficient is measured using low solute concentration, where K or Ko/w is a very weak function of solute concentration. Extensive data for the partitioning of drugs between octanol and water has been tabulated through the years, Table 1.1. Drugs having values of K much greater than 1 are classified as lipophilic whereas those with K smaller than 1 indicate a hydrophilic.
Limitations of Distribution Law
- The selected solvent liquid pair must be immiscible with each other. Any mutual solubility must not affect the distribution of solute if left aside for enough time to separate.
- The experimental temperature must be maintained constant. As the temperature has an effect on the solubility of solute, any change in temperature during determinations may change the findings.
- The solute in question should be in the same molecular state in both solvents. If any chemical change is observed the concentration of species common to both solvents only should be considered.
- Solute must be present in both the solvent at low concentrations. At high concentrations of solutes, Nernst’s distribution law does not hold well.
- Samples should be withdrawn for analysis only after the achievement of equilibrium. Early equilibrium attainment can be possible by vigorous shaking.
Applications of Distribution Law in Pharmacy
- Partition coefficient first finds applications in medicinal chemistry and drug design.
- It has proved useful in other related areas such as drug absorption, bioavailability, toxicity, bioaccumulation, and metabolism. Although it appears that the partition coefficient may be the best predictor of absorption rate, the effect of dissolution rate, pKa, and solubility on absorption must not be neglected.
- Partition coefficient values are helpful in knowing the hydrophobic drug-receptor interactions.
- Partition coefficient help to understand the mechanism of preservative action of weak acids and determination of its optimum concentration for the effectiveness of action.
- From the partition coefficient general idea about the solubility of the drug in the solvent can be judged. It can be further useful in drugs solubility enhancement.
- For a series of compounds, the partition coefficient can provide an empiric handle in screening for biologic properties. For drug delivery, the lipophilic/hydrophilic balance has been shown to be a contributing factor for the rate and extent of drug absorption.
- Although partition coefficient data alone does not provide an understanding of in-vivo absorption, it does provide a means of characterizing the lipophilic/hydrophilic nature of the drug. Since biological membranes are lipoidal in nature the rate of drug transfer for passively absorbed drugs is directly related to the lipophilicity of the molecule.
- Partition coefficient values are helpful in the extraction of drugs from mixtures such as blood, urine, and crude plant extracts. Drugs depending upon their partition coefficient values extracts in organic or aqueous solvents. Efficient extraction is carried out by repeating steps several times.
- Partition coefficient has applications in drug separation by partition chromatography. This technique comprises of silica column soaked in water to which a mixture containing drugs is applied. A water-immiscible solvent such as hexane is allowed to flow through the column. The drugs in the mixture are partitioned into hexane in order of their partition coefficient. The drug having a high partition coefficient will partition first followed by other drugs in a mixture with lower partition coefficient values. Each drug can be collected separately.
- Partition coefficient values help to study structure-activity relationships for a series of compounds.
- To study the release of drugs from gels, ointments, and creams partition coefficient is a very important consideration.
Make sure you also check our other amazing Article on : Electrometric pH Determination