Aim: To determine the solubility of Sodium Chloride in water (gravimetric method of estimation).
Principle of Gravimetric Method
Principle: Mathematically solubility is expressed as the concentration of solute in a solution, which is in equilibrium with the solute (solid). That means the concentration of solute in a saturated solution is its solubility.
Though concentration can be expressed in many units, the solubility of solids in solvents is usually expressed in relation to the solute and solvent that make a saturated solution. The pharmacopoeial expression of solubility is the number of a milliliter of solvents in which 1 gram of solute will dissolve to make a saturated solution.
The simplest way of solubility determination is to determine the concentration of solute in a saturated solution and work out the quantity of solvent in volume and quantity of solute in weight to express solubility.
The concentration can be determined by gravimetric, titrimetric, or by any instrumental method of analysis.
Sodium chloride (NaCl) can be estimated by the simple gravimetric method.
Apparatus and Materials Required: Conical flask, weighing balance, evaporating dish, pipette, sodium chloride, and water.
Process:
- An increasing amount of sodium chloride is added to about 50 ml water in a conical flask with shaking until the solution is saturated and a part of the solid is left undissolved (around 20-gram sodium chloride is required).
- The solution is filtered and 10 ml of the filtrate is pipetted out into a tared (preweighed) evaporating dish.
- The dish containing 10 ml filtrate is weighed.
- The filtrate is evaporated to dryness and further dried at about 100°C in an oven. Then it is cooled and weighed. Drying is continued till constant weight is obtained
Observation:
Room Temperature: ____ o C
- Weight of empty dish in g: w1
- Weight of dish + 10 ml solution in g: w2
- Weight of dish + dry solution in g: w3
Calculation:
Weight of solute in 10 ml solution in g = W3 – W1
Weight of solvent in 10 ml solution in g = W2 – W3
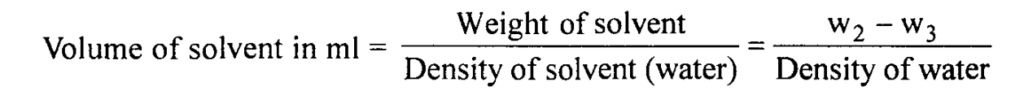
The density of water at room temperature can be referred from the appendix (for rough calculation density of water may be considered as 1 g/ml).
Solubility is the. parts of solvent required for 1 part of solute.
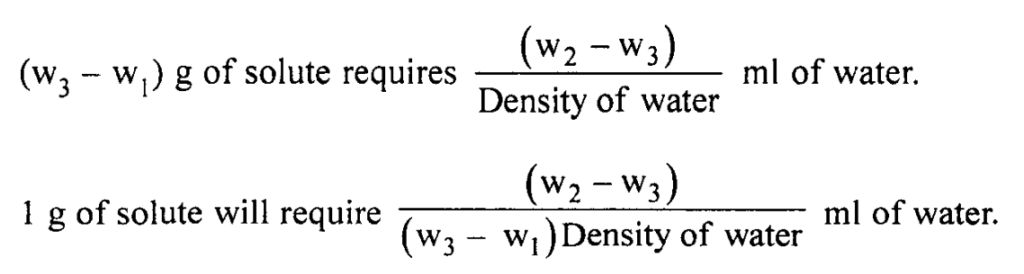
Report: The solubility of Sodium Chloride in water at room temperature (specify) is I in m\. (the solubility of sodium chloride is 1 in 2.8 ml water at 25 0C).
Make sure you also check our other amazing Article on: Sterile Powders