In the preparation of liquids, the drug is dissolved in the solvent of intended use. But the dissolution of drugs depends on;
- Nature of solvent
- Nature of solute
- The intensity of the forces present in solute-solvent
- solute-solvent interactions
- Steric factors
- Electronic factors
Table of Contents
Determination of Solubility
Before attempting to formulate a solution the solubilities of any ingredients must be determined. This can be done by placing an excess of the finely divided drug in a tightly closed container along with the solvent. This container is then agitated at a constant temperature bath for 72 hours. Samples are withdrawn to determine the solubility of the drug by suitable analytical technique.
Factors Influencing Solubility
pH:
Most of the drugs are either weak acids or weak bases. They are insoluble or slightly soluble in water while their salts are soluble. The solubility of these agents is markedly influenced by the pH of their environment.
The values for the solubility constant Ks and the dissociation constants Ka or Kb that are reported in the literature are usually for the drug in distilled water. These values are not always helpful as such. Because these values differ for the dosage formulation like elixir containing more amount of solid and cosolvents. In general, cosolvents such as alcohol or glycerin have the effect of increasing the solubility constant and decreasing the dissociation constant.
The drug’s pH environment should be fixed by keeping certain points in mind.
- The solvent used for the dosage formulation.
- The concentration of the drug required in the formulation.
- pH should not decrease the stability of the product.
- pH should encourage physiologic compatibility.
During the formulation of solutions of acidic and basic drugs, the following factors must be considered.
- The solubility of ionized and unionized forms of the drug.
- The chemical stability of the drug is a function of the pH and the buffer components.
- The therapeutic or pharmaceutical efficacy of the drug.
The buffer pH and buffer type are selected to maintain a proper balance between these three variables.
Buffers:
After fixing the pH of the drug’s environment, the formulation must be supplied in such a way as to maintain the pH throughout its life. But in certain cases, there is a possibility of change in the pH. To avoid the consequences of pH change, suitable buffers must be added. The selection of a buffer must be consistent with the following criteria:
- The buffer must have adequate capacity in the desired pH range.
- The buffer must be biologically safe for the intended use.
- The buffer should have little or no deleterious effect on the stability of the final product.
- The buffer should permit acceptable flavoring and coloring of the product.
- The pKa of the acid used in the buffer should be close to the desired pH of the final dosage form.
Typical buffer systems used in pharmaceutical preparations are acetate-acetic acid, bicarbonate-carbonate, borate-boric acid, and Na2HPO4-Na2H2PO4.
Cosolvency:
The solubility of certain drugs in water is insufficient. In such cases, their solubility is increased by using water-miscible solvents in which the drugs are soluble. This process is known as cosolvency and the solvents used for the purpose are known as cosolvents.
Mechanism: (i) Modification of polarity of the solvent system in such a way as to approach the polarity of the solute. (ii) The formation of a completely new solvent whose interactions cannot be easily predicted from the interactions of the individual components of the solvent mixture.
It is easy to predict solubilities of nonpolar or semipolar compounds in 20% ethanol in water than to predict solubilities of the same compounds in the water-ethanol-glycerin sorbitol solvent system. The sum of solubility values in individual solvents is usually not equal to its solubility in a blend of solvents.
Auxiliary use: Cosolvents are also used to facilitate the incorporation of volatile oils in the formulations to impart odor.
Examples: Cosolvents used in liquid orals are ethanol, sorbitol, glycerin, syrups, propylene glycol, PEGs, etc.
If ethanol is used as a cosolvent, then the amount of ethanol is kept to a minimum because of its pharmacological effect, burning taste in high concentration, and cost.
Dielectric Constant:
The well-known rule “like dissolves like” is based on the observation that molecules of similar charge distribution are mutually soluble. Molecules which have asymmetric charge distribution, i.e. polar molecules, are soluble in polar media, while non-polar molecules can easily be placed in non-polar media.
The dielectric constant is a measure of the polarizability of a molecule. Compounds are often classified according to their dielectric constants as polar, semipolar, or nonpolar.
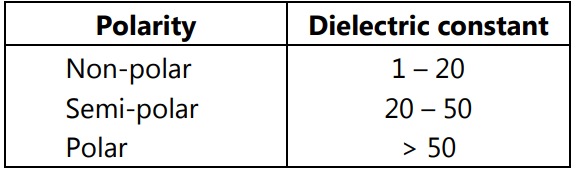
It should be remembered that the dielectric constant is not necessarily an accurate and sufficient predictive tool for solubility. The dielectric constant for sucrose is 3.3, yet it is very soluble in water. The dielectric constants of dioxane (2.26) and mineral oil (2.5) are essentially similar, yet dioxane is completely miscible with water whereas mineral oil is immiscible with water.
The dielectric constant of water decreases with an increase in temperature, yet the solubility of most compounds increases with an increase in temperature. Therefore solubility cannot be predicted from a combination of dielectric constant alone.
The dielectric constant of a combination of two solvents lies between the values for the individual components. The solvent properties of solvent blends having the same dielectric constant are not similar. The dielectric constant for 70% w/w ethanol is 61.1 which is very close to the dielectric constant for 40% w/w sucrose 59.9%, yet these two solvents have different solvent properties.
The dielectric constant of a solvent, therefore, gives only a rough qualitative prediction of the solvent properties and the degree of solubility of polar and non-polar compounds.
Solubilization:
Solubilization is an alternative method for increasing the solubility of poorly water-soluble drugs. Solubilization is defined as the spontaneous passage of poorly water-soluble solute molecules into an aqueous solution of soap or a detergent, in which a thermodynamically stable solution is formed.
When surfactants are added to a liquid at low concentrations, they tend to orient at the air-liquid interface. With the increased concentrations of surfactant, the molecules are forced into the bulk of the liquid. At still higher concentrations, the molecules of surfactant in the bulk of the liquid begin to form micelles. The concentration of surfactant at which it occurs is known as the critical micelle concentration (CMC).
Solubilization effects when micelles entrap or adsorb solute molecules. Therefore, the surface-active agent must be present in the solution at or above the CMC. For oral preparations, polysorbates are used as surfactants. For example, (1) cresol with a soap solution in which cresol solubility is increased with increased CMC formation (2) Cholesterol is more soluble in aqueous soap solutions than in pure water.
Complexation:
Organic compounds in solution generally tend to associate with each other to some extent. Every substance has specific, reproducible equilibrium solubility in a given solvent at a given temperature. Any deviation from this inherent solubility must be due to the formation of new species (complexes) in the solution.
Insoluble compounds often interact with a soluble ingredient to form a soluble complex. For example, preparation of iodine solutions. Insoluble iodine (I2) reacts with soluble I− of KI to form a soluble KII2 soluble complex.
In the case of weakly acidic and basic compounds, the total solubility is equal to the inherent solubility of the undissociated compound plus the concentration of the dissociated species. Similarly, when complex formation occurs, the total solubility is equal to the inherent solubility of the uncomplexed drug plus the concentration of drug complex in solution.
In utilizing this approach for increasing the solubility of a compound, one has to make sure that the complex is reversible, dissociates easily, and releases the active ingredient, otherwise, the active ingredient becomes therapeutically ineffective.
Hydrotrophy:
The term hypertrophy has been used to designate the increase in solubility in water of various substances due to the presence of large amounts of additives.
Mechanism: A complex is formed between solute and hydrotropic agent by the weak interaction.
Limitations:
- A large amount (20 to 50%) of additive is necessary to produce the effect.
- The extent to which solubility is increased is not sufficient.
- Many of the complexing agents are either physiologically active substances or are of unknown biologic character.
Examples:
- Sodium benzoate increases the solubility of caffeine.
- Sodium acetate increases the solubility of theophylline.
- Sodium benzoate increases the solubility of benzoic acid.
- PVP increases the solubility of iodine.
Chemical Modification of the Drug:
The solubility of poorly soluble drugs can be increased by the chemical modification of the drug.
Example: The solubility of betamethasone alcohol in water is 5.8 mg/100 ml at 25OC. The solubility of chemically modified drug betamethasone alcohol 21 disodium phosphate in water is 10 g/100 ml.
Limitation: Chemically modified drugs must be subjected to the prolonged testing protocol as the parent compound, including biologic activity studies, acute and chronic toxicity, pharmaceutical evaluation, and clinical testing. This procedure is time-consuming and expensive. Therefore this procedure is approached only when other procedures are not possible.
Temperature:
The solubility of most of the drugs/additives increases with the increase in temperature.
Salting-out:
The addition of large amounts of highly soluble salts to aqueous solutions of organic compounds often leads to precipitation or separation of organic solutes. This phenomenon is called as salting out and is attributed to competition between the salts and the organic compounds for solvent molecules (water). The salting-out power of a salt depends on the size and relevance of its ions.
Salting-in: This is a process of increasing the solubility of an organic compound upon the addition of salt. For example, salting-in of globulins (proteins) in water in presence of salts.
Particle Size:
Solubility increases with a decrease in particle size at submicron levels, an increase of about 10% in solubility is then observed. This is due to the large surface free energy associated with small particles.
Molecular Size of Solvent Molecules:
The solvent properties of water are due to the small size of its molecules. Any other liquids which are similar to water in polarity, dielectric constant, and hydrogen-bonding may be poor solvents for ionic compounds. The reason is because of the larger size of the liquid molecules when compared to water molecules. It is difficult for these liquid molecules to penetrate and dissolve the crystals.
Molecular Shape of Solute Molecules:
The shape of the solute molecule influences the solubility. The high solubility of ammonia in water is due to its shape which fits without difficulty in the structure of water. The effect of the shape of the solute molecules on their solubility in a given solvent is predominantly an entropy effect.
Macromolecules:
These are compounds with molecular weights ranging from 10,000 to millions. Examples are plasma proteins, enzymes, natural polysaccharides, cellulose derivatives, PVP, carbapol, etc. The solubility behavior of macromolecules depends on molecular weight ionic character, shape, pH, temperature, and added salts. Aqueous solutions are generally highly viscous and many form gels at low concentrations. These properties are the basis for the extensive use of macromolecules as a thickening and suspending agent in dosage forms.
Make sure you also check our other amazing Article on : Liquid orals
