Introduction To Acid-Base Titration
Acid-base titrations are also called neutralization or aqueous acid-base titrations. These titrations involve basically the reaction of H3O+ in solution against OH–. This basic reaction is applied to any acids and bases including strong acids, strong bases, weak acids, weak bases, salts of a weak acid and salts of weak bases. For example: In the following molecular form reactions various acids are reacted with bases.
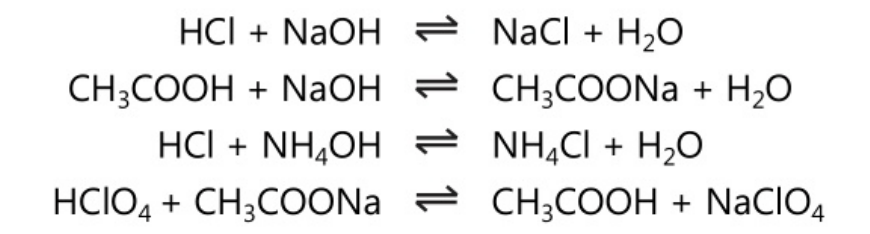
However, the basic reaction involved in the above-mentioned reactions is given below.
H_3O^+\;+\;OH^-\;\rightleftharpoons \;2H_2O
Thus, in acid-base titrations, the above reaction is effected using prescribed standard conditions of volumetric titrations. The endpoint is detected using the suitable indicator.
Theories of Acids and Bases
The theories of acids and bases have been postulated by describing the acid-base properties of the substances in water as well as in other solvents.
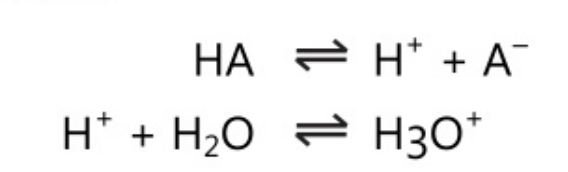
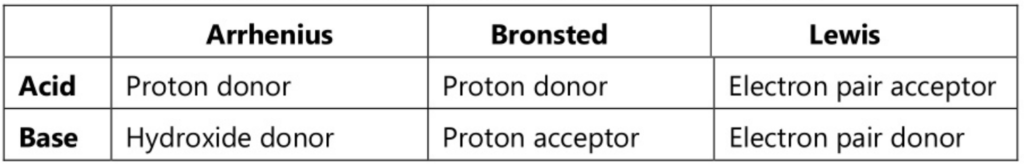
The first theory was postulated by Arrhenius in 1884 as a part of the general theory on electrolytic dissociation. As per this theory, acid is defined “as a substance which generates hydrogen ions when dissolved in water and further these hydrogen ions in association with solvent form hydronium ions.
Similarly, a base is defined as a substance that ionizes to give hydroxyl ions when dissolved in water.
BOH\;\rightleftharpoons \;B\;+\;OH^-
Substances in which OH ions are not present are not fit into the above definition. However, if the reaction leads to an increase in the OH– ion concentration of the solution, e.g. amines, then they are included as bases. Such substances are said to be ‘pseudo base’ on the basis of hydrolysis or the reaction with water to produce hydroxyl ions.
B\;+\;H_2O\;\rightleftharpoons BH^+\;OH^-
This theory explains the quantitative acid-base behaviour in aqueous solution but does not account for acid-base behaviour in non-aqueous solvents.
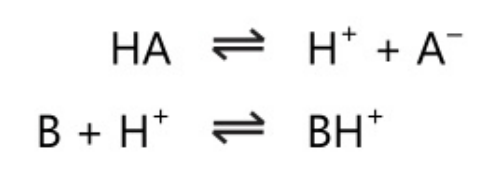
Bronsted proposed the new theory in 1923; Lowry also proposed a similar theory independently. He defined an acid as a species that can donate protons’ and a base as a species that can accept protons’.
In Bronsted theory, an acid donates a proton. It is independent of solvent. This theory differs from Arrhenius’s theory in its concept of the base. In the system,
HA\rightleftharpoons \;H^+\;+\;A^-
The anion A– acts as a base as it accepts a proton. Also in other systems,
B\;+\;H^-\;\rightleftharpoons \;BH^+
The cation BH+ acts as an acid as it donates a proton. The acid and base pairs in the above reaction are conjugate acid-base pairs, ie. the base is the conjugate base of the acid and the acid is the conjugate acid of the base.
Acid\;\rightleftharpoons\;Base\;+\;H^+
For the acid-base reaction, it is necessary to have two conjugate acid-base systems as it involves proton transfer from one system to the other; Bronsted acid-base theory explains the acid-base reactions in various solvents.
The conjugate acid-base system is essential to observe a reaction which can be considered a proton transfer from one system to another:
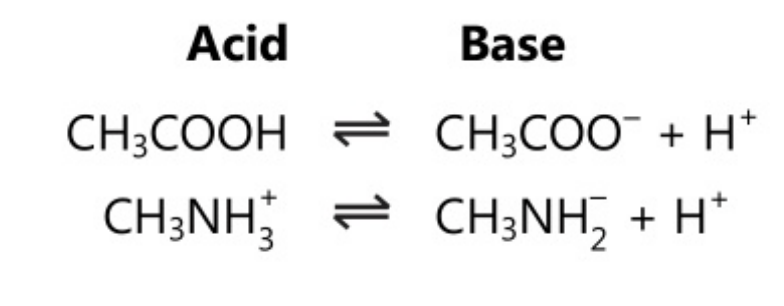
It can also be noted as
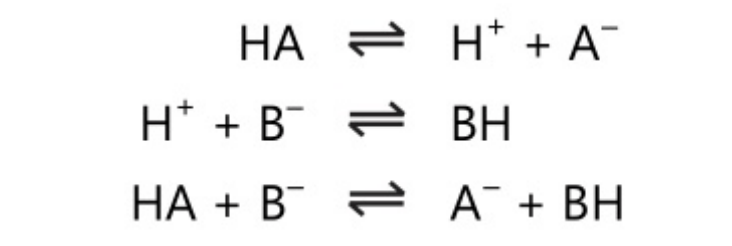
Some solvents like water can act as an acid-base pair as they have acid-base properties. Water can act as both acid and base, i.e. it is amphoteric in nature.
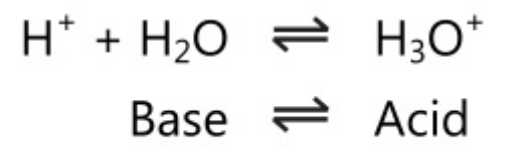
This dual behaviour of water allows it to act as a second conjugate acid-base system for solutes that are either acids or bases.
Lewis proposed a theory in 1923, called as Lewis theory. This theory describes an acid as a species which has the capability to accept an electron pair whereas a base is described as a species which has the capability to donate an electron pair. There is no change in the concept of acid-base as every proton acceptor is an electron-pair donor.
This theory is useful to describe the indicator colour change in non-protonic systems exhibiting acid-base reactions.
The following chart will simplify all three theories about acids and bases.
Make sure you also check our other amazing Article on: Limit Tests
