Table of Contents
What is the limit test in pharmaceutical analysis?
Limit tests are defined as, quantitative or semi-quantitative tests which are performed to identify and control a small quantity of impurities which are likely to be present with the substances to be analysed. Generally, limit tests are carried out to identify the inorganic impurities present in pharmaceutical substances.
In these tests, the turbidity/opalescence/colour intensity produced by the test sample is compared with the turbidity/opalescence/colour intensity produced by the standard.
For instance, the principle of the limit test for chlorides and sulphates is based upon the measurement of opalescence or turbidity produced in the known amount of substance (by addition to reagent), and comparing it with the standard opalescence or turbidity. For comparison of turbidity for different substances with varying amounts of an impurity, the amount of substance to be used is varied, and not the standard turbidity. Pharmacopoeia does not give a numeric value to the limits, as it is not practicable as its content will be influenced to a great extent by large quantities of other substances present.
In performing limit tests, it is essential to follow the directions indicated by Pharmacopoeia.
Preparation of the Solution for Tests:
A specified amount of the substance is dissolved in distilled water, and the volume is made to 50 ml in a Nessler’s cylinder. Depending upon the nature of the substance, some modifications are carried out for the preparation of the solution: alkaline substances like carbonates, hydroxides etc. are dissolved in sufficient quantity of acid so that effervescence ceases, and free acid is present. For insoluble substances like kaolin, a water extract is prepared, filtered and then the filtrate is used. Salts of organic acids like sodium benzoate, sodium salicylate etc., liberate free water-insoluble organic acid, during acidification which is filtered off and the filtrate is used for the test. Coloured substances like crystal violet, malachite green etc. are carbonized and the ash so produced is extracted in water. Reducing substances like nitrite, hypophosphate etc., are oxidized with oxidizing agents, and the solution is prepared and used. Substances like potassium permanganate are reduced by boiling with alcohol, and the filtrate is used.
Limit Test for Chloride
The limit test for chlorides is based on the chemical reaction between soluble chloride ions with a silver nitrate reagent in a nitric acid media. The insoluble silver chloride renders the test solution turbid (depending upon the amount of silver chloride formed and therefore, on the amount of chloride present in the substance under test.) This turbidity is compared with the standard turbidity produced by the addition of silver nitrate, to the known amount of chloride ion (sodium chloride) solution. If the test solution shows less turbidity than the standard, the sample passes the test.
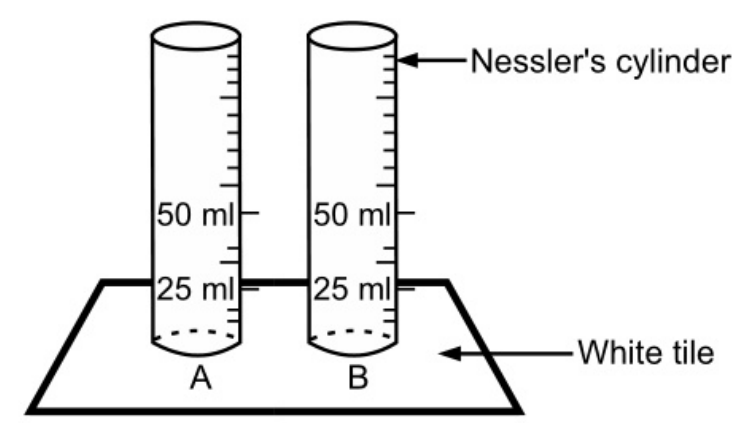
Dissolve the specified quantity of substance in water or prepare a solution as directed in the pharmacopoeia and transfer it to Nessler’s cylinder A (Fig. 1.1). Add 1 ml of dilute nitric acid except when nitric acid is used in the preparation of the solution. Dilute it to 50 ml with water and add 1 ml of silver nitrate solution, stir immediately with a glass rod and set aside for 5 minutes.
Simultaneously for standard opalescence, place 1 ml of 0.05845 per cent w/ solution o sodium chloride in Nessler’s cylinder B and add 10 ml of dilute nitric acid, make up the volume to 50 ml with water, add 1 ml of silver nitrate solution, stir with the glass rod and set aside for 05 minutes. The opalescence produced by the sample (in cylinder A) should not be greater than standard opalescence.
Limit Test for Sulphate
In a limit test for sulphate, the solution of the substance under test is mixed with barium sulphate reagent in a hydrochloric acid medium and the turbidity so produced is compared with the standard in a similar manner with a known quantity of sulphate ion (using potassium sulphate). The substance passes the limit test if it produces a turbidity that is less than the standard.
A solution of the specified quantity of the substance is made in water or prepared as directed in the pharmacopoeia in Nessler’s cylinder; add 2 ml dilute hydrochloric acid except where hydrochloric acid is used in the preparation of the solution. Dilute it to 45 ml with water, add 5 ml of barium sulphate reagent, stir immediately with the glass rod and set aside for 5 minutes. To produce standard turbidity, place 1 ml of 0.1089 per cent w/v solution of potassium sulphate and 2 ml of dilute hydrochloric acid in another Nessler’s cylinder, dilute to 45 ml with water, add 15 ml of barium sulphate reagent, stir immediately and set aside for 5 minutes. The turbidity produced by the sample solution is not greater than the standard turbidity.
British Pharmacopeia makes use of a barium sulphate reagent, which contains barium chloride, alcohol and a small amount of potassium sulphate. Alcohol prevents super-saturation, and potassium sulphate increases the sensitivity of the test by giving the ionic concentration in the reagent, which just exceeds the solubility product of barium sulphate.
Limit Test for Iron
Limit Tests is based upon the reaction of iron in an ammoniacal solution, with thioglycolic acid which forms a pink to a deep reddish purple-coloured complex of iron-thioglycollate. The colour produced from a specified amount of substance from the test is compared by viewing vertically, with a standard (Ferritic ammonium sulphate). If the colour from the test solution is less dark than the standard then the sample passes the test.
The Fe (SCH2COOH)2, formed with the ferrous form of iron, is quite stable for long period in the absence of air. The colour, however, is destroyed by oxidizing agents and strong alkalis. The original state of iron is unimportant, as thioglycolic acid reduces Fe3+ to Fe2+. This test is very sensitive. Interference with other metal cations is eliminated, by making use of 20 per cent citric acid, which forms complex with other metal cations.
Method:
Prepared solution by dissolving a specified amount of substance in 40 ml water or taking 10 ml of solution as directed in the monograph in Nessler’s cylinder. Add 2 ml of 20 per cent w/v solution of iron-free citric acid and 0.1 ml thioglycolic acid, mix and make alkaline with iron-free ammonia solution and dilute it to 50 ml with water. Allow standing for 5 minutes. For standard, simultaneously dilute 2 ml of standard iron solution with 40 ml of water, and add the same quantity of reagent as in the sample. Any colour produced by the sample is not more intense than the standard.
Earlier, ammonium thiocyanate reagent was used for the limit test of iron. Since thioglycolic acid is a more sensitive reagent for iron, it has replaced ammonium thiocyanate in the test.
Limit Test for Heavy Metals
Besides the limit test for lead, the Indian Pharmacopoeia and US include limit tests for heavy metals present in many compounds. Lead and other heavy metals are generally found as impurities in pharmaceutical substances. Two separate tests are therefore prescribed by these pharmacopoeias.
The limit test for heavy metals is based upon the reaction of the metal ion with hydrogen sulphide, under the prescribed conditions of the test resulting in the formation of metal sulphides. These remain distributed in a colloidal state and produce a brownish colouration.
The test solution is compared with a standard prepared using a lead solution (as the heavy metal). The metallic impurities in substances are expressed as parts of lead per million parts of the substance. The usual limit as per I P is 20 ppm.
Heavy Metals:
The Indian Pharmacopoeia adopts three methods for the limit tests for heavy metals. The
‘Method A’ is used for the substance which yields a clear colourless solution under specified conditions. ‘Method B’ is used for those substances which do not yield a clear colourless solution under the test conditions specified for method A. ‘Method C’ is used for substances that yield a clear colourless solution in sodium hydroxide medium. The reagents like acetic acid, ammonia, hydrochloric acid, nitric acid, potassium cyanide and sulphuric acid should be lead-free and designated as ‘Specific reagents’.
Method A:
The standard solution is prepared by taking 2 ml of standard lead solution and by diluting it to 25 ml with water. The pH is adjusted between 3 and 4 by using either dilute acetic acid or a dilute ammonia solution. Make up the volume of 35 ml with water.
The test solution is prepared as directed in the individual monograph. Take 25 ml and adjust the pH of the solution between 3.0 and 4.0 by using dilute acetic acid or dilute ammonia and adjust the volume to 35 ml with water.
To each of the cylinders containing standard and test solution, add 10 ml of freshly prepared hydrogen sulphide solution, mix, dilute to 50 ml with water and allow it to stand for 5 minutes. The colour, when viewed downwards over a white surface, should not be darker for the test than the standard solution.
Method B:
The standard solution is prepared as directed under method A. Test solution is prepared in a crucible by weighing a specified quantity of substance as per the monograph. Moisten the substance with sulphuric acid, and ignite on a low flame till completely charred. Add a few drops of nitric acid and heat to 500°C. Allow to cool, add 1 ml of hydrochloric acid and evaporate to dryness. Moisten the residue with 10 ml hydrochloric acid and digest for two minutes. Neutralize with ammonia solution and make just acidic with acetic acid. Adjust the pH between 3.0 and 4.0, and filter if necessary. Adjust the volume of the filtrate to 35 ml in Nessler’s cylinder, add 10 ml of hydrogen sulphide solution, dilute to 50 ml with water and compare the colour with the standard solution.
Method C:
The standard solution is prepared by using 2 ml of standard lead solution; adding 5 ml dilute sodium hydroxide solution and making the volume to 50 ml with water. For the test solution, take either 25 ml solution prepared as directed in the monograph or dissolve a specified quantity of substance in 20 ml water, add 5 ml of dilute sodium hydroxide solution and make up the volume to 50 ml.
To each of the above solutions in Nessler’s cylinder add 5 drops of sodium sulphide solution, mix and set aside for 05 minutes. The colour produced by the test solution should not be darker than the standard solution.
Limit Test for Volatile Oils
In 25 ml glass, stoppered test tubes; 10 ml of the oil is mixed with an equal volume of water containing a drop of hydrochloric acid. Hydrogen sulphide is passed through the mixture until it is saturated. No darkening in colour should be produced neither in the oil nor in the water layer, for the sample to pass the test.
Limit Test for Lead
The limit test for lead as per IP and USP is based upon the reaction between lead and diphenyIthiocarbozone (dithizone). Dithizone in chloroform extracts leads from alkaline aqueous solutions as a lead Dithizone complex (red in colour).
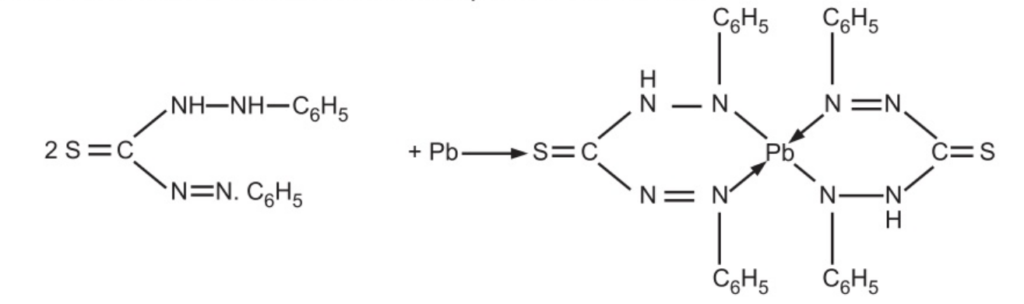
The original dithizone has a green colour in chloroform thus the lead-dithizone shows a violet colour. The intensity of the colour of the complex depends upon the amount of lead in the solution. The colour of the lead-dithizone complex in chloroform is compared with a standard volume of lead solution, treated in the same manner.
In this method, the lead present as an impurity in the substances is separated by extracting an alkaline solution with a dithizone extraction solution. The interference and influence of other metal ions etc. are eliminated by adjusting the optimum pH for the extraction, by using ammonium citrate, potassium cyanide, hydroxylamine hydrochloride reagents etc.
Method:
A known quantity of the sample solution is taken in a separating funnel: 6 ml of ammonium nitrate, and 2 ml of hydroxylamine hydrochloride are added, followed by 2 drops of phenol red, and the solution is made alkaline by adding an ammonia solution. Add 2 ml of potassium cyanide solution and extract immediately with 5 ml portions of dithizone solution (till green). The combined dithizone extracts are shaken for 30 seconds, with 30 ml of 1 per cent nitric acid, and the chloroform layer is discarded. To the acid solution, 5 ml of standard dithizone solution is added along with 4 ml of ammonium cyanide and shaken for 30 seconds. A known quantity of the standard solution of lead (equivalent to the amount of lead permitted in the sample) is treated separately. The violet colour of the chloroform layer- of the sample should not be darker than the standard for the sample to pass the test.
In the preparation, appropriate preliminary treatment is given to get lead in the solution, without any interfering substance or ion. All reagents employed under the test (except for standard lead solution), should be free from lead and are designated as ‘PbT’ reagents in pharmacopoeias.
Limit Tests for Lead as per British Pharmacopeia:
British Pharmacopeia adopts another method for the limit test for the lead which is based on the formation of a brownish colouration produced by the colloidal lead sulphide upon the addition of sodium sulphide to the solution under test. If the lead content is more, then a brownish-black precipitate of lead sulphide is obtained. The colour produced in the test solution is matched against the standard that is made from a known amount of lead in a ‘Nessler’s cylinder. In order to carry out this test two solutions, a primary and an auxiliary are prepared from the sample.
Method:
Two solutions of the substance under test are prepared with hot water and acetic acid.
One is the primary solution containing a definite but greater amount of substance and placed in a 50 ml Nessler’s cylinder. The other is the auxiliary solution containing a known amount of the test substance in another 50 ml Nessler’s cylinder. To this auxiliary solution, a definite amount of a dilute solution of lead nitrate is added. Ammonia and potassium cyanide solutions are added to both the solutions in the Nessler’s cylinders. Small amounts of burnt sugar solution are added to both solutions; to correct any difference in colour and the volume is made up to 50 ml. If the solutions appear turbid, they are filtered and the volume is made up to 50 ml. Both solutions are treated with sodium sulphide solution and a colour is developed. If the colour in the auxiliary solution is darker than that in the primary, the substance contains lead within limits.
The object of using primary and auxiliary solutions of substances is to have a comparison made under identical conditions. Interference by any unknown entity present in the solution is eliminated by this technique.
Limit Test for Arsenic
Arsenic is an undesirable and harmful impurity in medicinal substances, and all pharmacopoeias prescribe a limit test for it. There are many qualitative and quantitative tests for arsenic. The pharmacopoeial method is based on the Gutzeit test. In this test, arsenic is converted into arsine gas, (AsH3) which when passed over a mercuric chloride test paper, produces a yellow stain. The intensity of the stain is proportional to the amount of arsenic present & a standard stain produced from a definite amount of arsenic is used for comparison.
The chemical reactions involved in the method are given below:
When the sample is dissolved in acid, the arsenic present in the sample is converted to arsenic acid. The arsenic acid is reduced by reducing agents like potassium iodide, stannous chloride etc. to arsenious acid.
H_2AsO_4\rightarrow H_3AsO_3
The nascent hydrogen produced during the reaction further reduces arsenious acid to arsine (gas), which reacts with mercuric chloride paper, producing a yellow stain.
\underset{arsenious\;acid.}{H_3AsO_3}+\;3H_2\rightarrow\underset{Ar\sin e\;(gas)}{AsH_3\;2H_2O}
To carry out the test, a specified apparatus (as described in pharmacopoeias) is used. In order to convert arsenic into arsine gas, various reducing agents like zinc and hydrochloric acid, stannous chloride and potassium iodide are employed. The rate of evolution of gas is maintained by using a particular size of zinc, and controlling the concentration of acids and other salts of the reaction medium, besides temperature. Any impurity coming along with the gas (like H2S) is trapped by placing a lead acetate-soaked cotton plug in the apparatus. All the reagents employed for the test should be arsenic-free and are designated as AsT in pharmacopoeias.
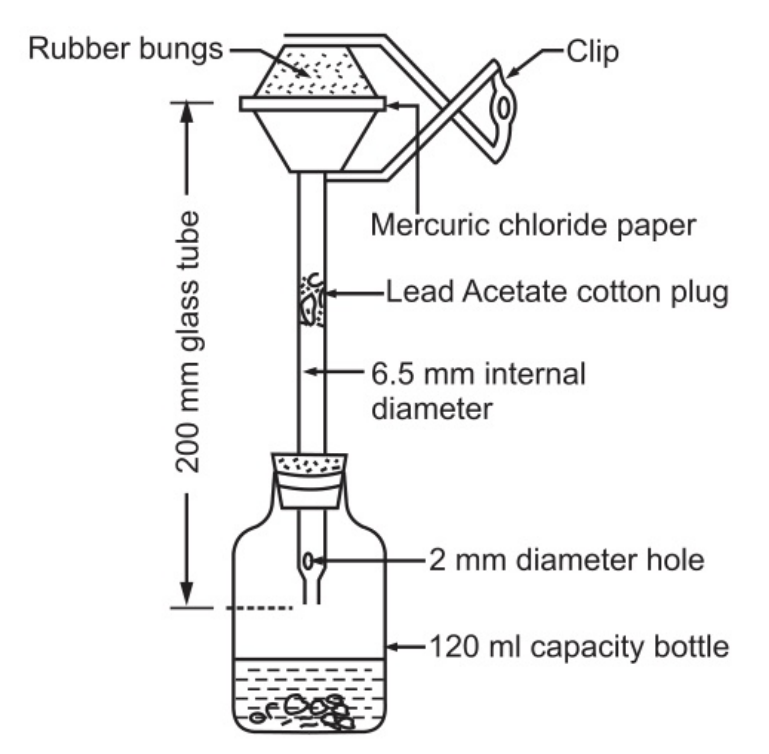
Apparatus:
An apparatus shown in Fig. 1.3 as per the specification of I P is used for the limit test for arsenic.
A wide-mouth bottle of 120 ml capacity fitted with a rubber bung carrying a glass tube 200 mm long and 6.5 mm internal diameter with a hole of 2 mm at one end is used in the test. The other end of the glass tube is cut smooth and carries rubber bungs (25 × 25 mm). Mercuric chloride paper is sandwiched between the rubber bungs. The rubber bungs are held in place by means of a clip.
General Procedure:
A solution of the substance as specified in the monograph is placed in the generator bottle. Potassium iodide 1 g and 10 g of zinc AsT are added. Mercuric chloride test paper is placed in the rubber slit and the stopper is placed in the position immediately. It is set aside for 40 minutes and the stain produced on the paper is compared with the standard stain. The standard stain is produced simultaneously by taking 50 ml of water; 10 ml of stagnated HCl and dilute arsenic solution varying from 0.02 ml – 1.0 ml. (1 ml = 0.01 mg of arsenic). If the sample shows a stain of lesser intensity than that of the standard, then it passes the test. For example, if 1 g of a substance under test is compared with 0.01 ml of dilute arsenic solution, on matching will contain 1 pm arsenic in the sample.
The stain produced on paper fades on keeping, and therefore, the comparison should be made immediately. Stained papers can be preserved by dipping them in hot melted paraffin, and keeping them away from light. In order to get reproducibility of the results, it is essential to follow the directions given in pharmacopoeia.
Modification of the general method of testing is carried out for certain substances. This is to have arsenic in the final solution in a readily reducible form. The interference of other substances, ions etc. is eliminated by preliminary treatment. For example, carbonates, hydroxides and oxides give effervescence, so brominate hydrochloric acid is used. Nitrates are heated with sulphuric acid, to expel nitric acid. Certain organic compounds are insoluble in acid and water and cause frothing. Hence, organic matter is removed by igniting with calcium hydroxide. Solutions of organic acids like citric, tartaric etc., are prepared in stagnated hydrochloric acid. Iron, bismuth and antimony salts are taken in 20 per cent HCI and distilled. Dyes and related compounds are decomposed with sulphuric acid (after preliminary treatment with nitric acid) and then used
The British Pharmacopoeial method is similar to the IP method. The apparatus and design are slightly different. The amounts of zinc, hydrochloric acid and other reagents employed are also different. Further, BP adopts the use of mercuric bromide test paper. The Gutzeit test for arsenic is very sensitive and hence, is adopted by pharmacopoeias of various countries.
Make sure you also check our other amazing Article on: Sources of Impurities
