Table of Contents
BODY ODOUR
When the body gives off a scent others may find difficult, known as body odor. Body odor is the perceived unpleasant smell our bodies can give off when bacteria that live on the skin break down sweat into acids.
Some say it is the smell of bacteria growing on the body, but it is actually the result of bacteria breaking down protein into certain acids.

Odor is caused by the combination of sweat and bacteria normally found on the skin.
CAUSES OF BODY ODOUR
- The body is covered with sweat glands because sweating is an essential function that helps us cool down.
- There are two main kinds of sweat glands viz., eccrine and apocrine. Eccrine glands cover much of the body and open directly on the skin‟ ‘s surface.
- By contrast, apocrine glands occur in areas that contain a lot of hair follicles, like the groin and armpit. Ø When the body heats up, eccrine glands release sweat that cools your body. It is typically odorless until bacteria on your skin start breaking it down.
- Certain foods and drinks you have consumed, as well as certain kinds of medication, can also cause eccrine sweat to smell.
- Apocrine glands work primarily under stress, secreting an odorless fluid. This fluid begins to develop an odor when it encounters bacteria on the skin.
- Diabetes
- Being overweight
- Hyperhidrosis
- Kidney and liver dysfunction
- Bromhidrosis
- Excessive sweating
- Foods
- Spicy food
- Thyroid conditions
ANTIPERSPIRANTS
Definition Of Antiperspirants
In a regulatory monograph the FDA, through the Food Drug and Cosmetic Act, defines antiperspirants as an over-the-counter (OTC) drug when applied topically to reduce production of underarm sweat (perspiration).
As per the Department of Health and Human Services, antiperspirant is a drug product applied topically that reduces the production of perspiration (sweat) at that site”.
In general, antiperspirants defined as topically applied, OTC products, cosmetic product, or cosmeceutical products designed to reduce the wetness at the application site or underarm wetness to reduce the secretion of sweat or reduces the production of perspiration (sweat) by limiting eccrine sweat production (penetrate the duct of the sweat gland, block it).

KEY ANTIPERSPIRANT ACTIVES
- Buffered Aluminum Salts (ACH): The first antiperspirant, based on AlCl3 introduced to the market in 1903, the first cream containing aluminum sulfate was introduced during the 1930s.
- The acidic pH value (2.5-3.0) was a drawback of these products, leading to skin irritation in the underarm pit.
- History tells us that the development of actives with a higher pH value, so-called buffered aluminum chlorides (aluminum chlorohydrate, ACH, pH= 4.0-4.2) was an appropriate step with the additional benefit of reduced destruction of fabric clothes.
- The formula of this buffering salt is {Al2 (OH)5} + + {Cl–}, or more conveniently Al2 (OH)5Cl.
Aluminum Zirconium Chlorohydrate-Glycine Complexes (AZG or ZAG)
- Aluminum zirconium chlorohydrate is obtained by reaction of ACH with zirconyl chloride. Reaction of the former ingredients in the presence of glycine leads to ZAG complexes.
- Classification of antiperspirant actives based on US FDA antiperspirant review panel
NON-SPRAY ANTIPERSPIRANTS
(a) Category-I (Permitted): Aluminum chloride up to 15% (calculated on hexahydrate form), Aluminum chlorohydrate up to 25%, Aluminum zirconium chlorohydrate, Buffered aluminum sulfate.
(b) Category-II (Prohibited): Aluminium bromohydrate (Safety, Efficacy), Aluminium chloride (alcoholic solutionsSafety)
(c) Category-III (Temporarily permitted): Aluminium sulphate, Potassium aluminum sulphate (Safety, Efficacy), Sodium aluminium chlorhydroxy lactate (Efficacy).
SPRAY ANTIPERSPIRANTS
(a) Category-II (Prohibited): Aluminium bromohydrate (Safety, Efficacy), Aluminium chloride (alcoholic solutions Safety), and Aluminum zirconium chlorohydrate (Safety)
(b) Category-III (Temporarily permitted): Aluminum chlorohydrate (Safety), Aluminium chloride (Safety), Aluminium sulphate (Safety, Efficacy), Buffered aluminium sulphate (Safety), Potassium aluminium sulphate (Safety, Efficacy), Sodium aluminum chlorhydroxy lactate (Safety, Efficacy).
Key antiperspirant actives
- Aluminum chlorohydrex polyethylene glycol up to 25%, Aluminum chlorohydrex propylene glycol up to 25%, Aluminum dichlorohydrate up to 25%, Aluminum dichlorohydrex polyethylene glycol up to 25%, Aluminum dichlorohydrex propylene glycol up to 25%, Aluminum sesquichlorohydrate up to 25%, Aluminum sesquichlorohydrex polyethylene glycol up to 25%, Aluminum sesquichlorohydrex propylene glycol up to 25%
- New concepts for controlling underarm wetness
- Titanium metal chelates
- Film-forming antiperspirant polymers
- Lyotropic liquid crystals
Mechanism of action of antiperspirants
- The mechanism of action of antiperspirant is the formation of gel plugs in sweat pores. This prevents sweat from emerging onto the skin surface, keeps the axillary dry, and eliminates the food source for the bacteria.
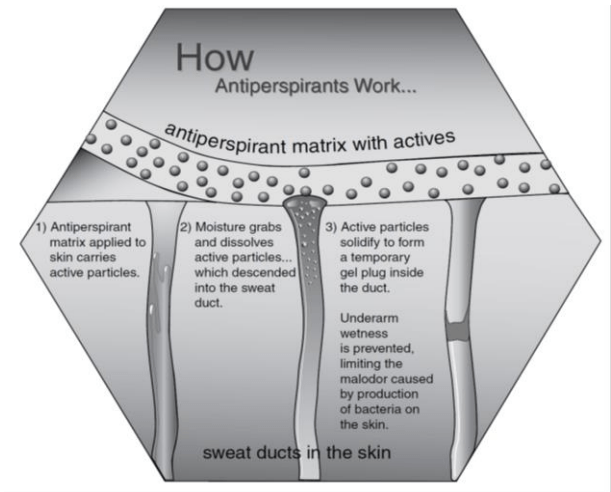
Antiperspirant formulations
Concept:
- Antiperspirant actives like ACH or ZAG complexes are soluble in water.
- The application of a concentrated aqueous solution of an antiperspirant gives a rather tacky feeling.
- Reduction of tackiness can be best achieved by silicone oils (cyclomethicones) or ester oils like Di-(2-ethylhexyl) adipate.
- The acidic pH value (pH 4.0- 4.2) has been taken into account by selecting additional components for the desired drug delivery system.
ANTIPERSPIRANT STICKS
Formula for suspension stick

Formula for clear anhydrous gel sticks

Formula for w/o emulsion stick
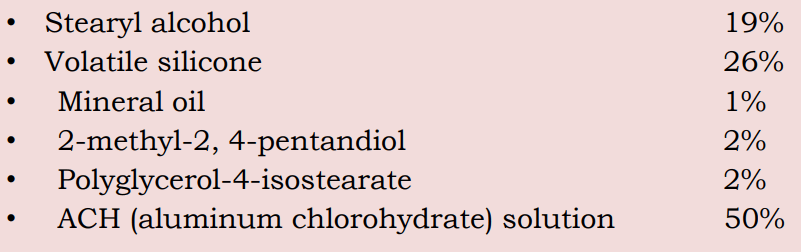
The formula of compressed Antiperspirant powder

DEODORANTS
Definition Of Deodorants
Deodorants are topically applied products designed to reduce underarm odor, prevent unpleasant odor, or minimize underarm axilla odor with an antimicrobial agent or a fragrance by adsorbing odor or masking the odor, feel fresh and odor-free.
Not all deodorants are antiperspirants, but all antiperspirants are automatically a deodorant due to the presence of actives in antiperspirants, which have bactericidal properties, rapidly reducing the indigenous bacterial population when applied regularly.

- Concepts and Actives used in deodorants to reduce underarm odor
- Antiperspirant Active-containing deodorants: Antiperspirant actives like aluminum chlorohydrate or the Al-Zr complexes reduce the secretion of eccrine sweat. The acidity of the aluminum salts may be a major factor in bacterial growth inhibition.
Odour quenching deodorants
- Zinc ricinoleate
- Metal oxides
- Esterase inhibitors
- Zinc glycinate
- Tri ethyl citrate
Antimicrobial active-containing deodorants
- Triclosan (2,4,4′-trichloro-2 ′-hydroxydiphenylether)
- Glyceryl fatty acid ester
- Sucrose fatty acid ester
- Glycerol ether
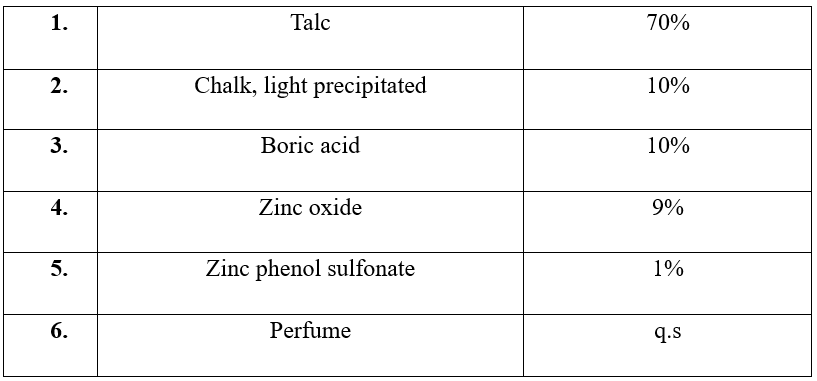
Formula for deodorant powder
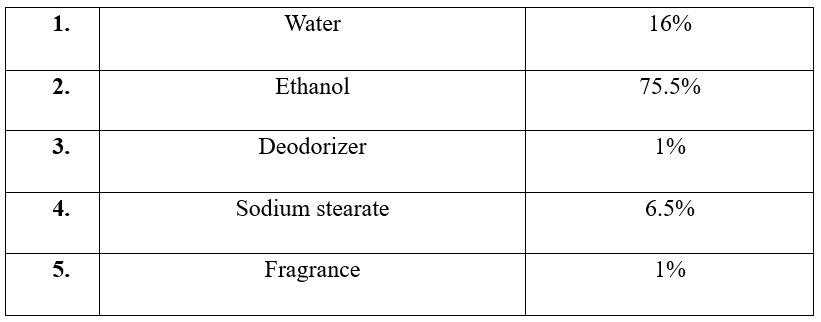
Formula for deodorant stick
Make sure you also check our other amazing Article on: Hair Colorants
