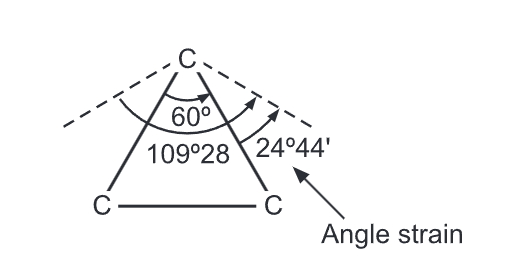
Baeyer Strain Theory: Since the carbon atom is tetrahedral in nature, the angle between any two bonds should be 109°28′. Thus any deviation from this value would result in an internal strain in the molecule. When an open chain organic compound is converted into a cyclic compound, a definite distortion of this normal angle of 109° takes place leading to a lot of strain in the molecule.
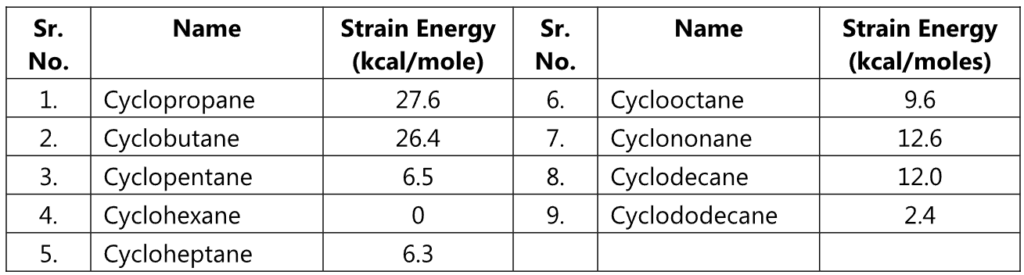
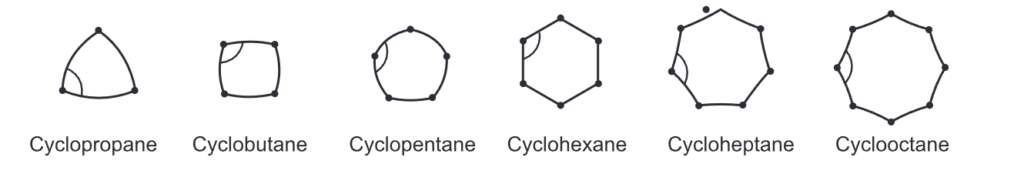
Small rings, such as three and four-membered rings have their bond angle (60° and 90° respectively) deviating much from the ideal angle of 109° of a tetrahedral carbon. Thus, these rings possess a significant amount of ring strain. A ring with 5 to 7 carbons is considered to have minimal to zero angle strain. Examples include cyclopentane, cyclohexane, and cycloheptane. However, a ring with 8 to 12 carbons possesses a moderate strain.
Ring strain (Banana Bond): In the case of greater ring strain, the bonds are curved. In the case of strain-free rings, the bonds are straight.
The molecular strain is the summation of
- Torsional distortion (Pitzer strain): It originates from the closeness of bulky substituents in a particular conformation
- Bond angle distortion (Baeyer strain): It originates from the deviation of bond angle from 109°28′, and
- Linear bond stretching or compression (Van der Waals strain)
In 1885, German chemist, Adolf Von Baeyer proposed that the stability of cycloalkane depends on the amount by which the angles between the chemical bonds deviate from the ideal angle 109°28′ of a tetrahedral carbon. The amount of deviation is the measure of the strain of the ring. The greater the strain, the less stable is the ring. Cyclopentane (bond angle is 108°) is the least strained and most stable ring. He assumed that all cycloalkane rings are planar. Hence, strain increases with the size of the ring.
Limitations of Baeyer Strain Theory
Baeyer postulated that the ring strain increases with the size of the ring. He assumed that all cycloalkane rings are planar. However, another German chemist, H. Sachse 1890 suggested that the ring strain in bigger-sized rings can be relieved if we place them in chair and boat conformations rather than in planner conformation. It is found to be practically correct.
He proposed that large rings need not be strained, because the carbons need not be coplanar. In 1918, 25 years after Sachse’s theory of strainless rings, Ernst Mohr proved its correctness on the basis of the X-ray structure of diamond using X-ray diffraction.
For example, the ring strain and ring stability of higher rings were in no way differ much from that of cyclopentane.
Coulson and Moffitt’s Modification:
Due to the triangular planar structure of cyclopropane, the bond angles between carbon-carbon bonds are expected to be 60°. It is very less than the ideal stable angle of 109° as per the sp3 hybridization of the carbon atoms. This leads to a considerable amount of ring strain in the molecule. Coulson and Moffitt suggested that rehybridization occurs in cyclopropane wherein the carbon-carbon bonds are bent outwards so that the bond angle becomes 104° which consequently reduces the level of ring strain. These bonds are called ‘bent bonds’ or ‘banana bonds’.
Thus, the C-C bonds have more p-character while the C-H bonds have more s-character. Hence, cyclopropane is much more reactive than alkanes or other higher ring systems.
Make sure you also check our other amazing Article on : Reaction of Phenanthrene