Basicity of Amines: Ammonia is the strong base. Amines are considered to be the alkyl or aryl derivatives of ammonia.
(i) Electron releasing nature of alkyl groups: The lone pair of electrons on the nitrogen atom of amines makes these compounds basic. The greater the availability of the lone pair electrons on nitrogen, the greater will basicity. It means basicity increases with increasing negative charge on nitrogen.
3º amine > 2º amine > 1° amine
Because alkyl groups donate electrons to the more electronegative nitrogen, the inductive effect makes the electron density of the alkyl amine’s nitrogen greater than the nitrogen of ammonia. Hence, we expect the tertiary alkyl amines are the most basic in the series.
But this is not so. In reality, the order of basicity is
2º amine > 1° amine – 3° amine > ammonia
This is because of
(ii) Steric hindrance: The size of an alkyl group creates a steric hindrance for the attack of a proton (H) to the lone pair of electrons present on the nitrogen. So, the more the number of alkyl groups attached, the lesser will be the basicity.
(iii) Solvation of amines: The basic strength of an amine in water is also governed by the extent to which the cation formed by uptake of a proton, can undergo solvation and so become stabilized. Tertiary amine cation has less solvation and so the stability. Hence, the introduction of a third alkyl group to form tertiary amine actually decreases the basic strength.
The number of hydrogen bonds possible when 1° amine is dissolved in water is the greatest, implying that they are the most stable amine, the least being 3º amines.
The combined effect of (i) electron releasing nature of alkyl groups, (ii) steric hindrance, and (iii) the solvation of amines causes the basicity order in the amines to be
2° amine > 1° amine 3° amine > ammonia
In aromatic amines, aniline is a very weak base. The base weakening effect is more pronounced with the attachment of further phenyl group to the nitrogen atom.
Aniline > Diphenyl amine > Triphenyl amine
Effect of Substituents on Basicity of Amines:
Aniline is the simplest aromatic amine. The effect of substituent on the basicity of aniline depends on the electron-withdrawing or donating nature of the substituent present at the ortho position to the NH2 group in aniline. Usually, electron-donating groups make the aniline more basic while electron-withdrawing groups make aniline less basic.
Ortho substituted anilines are less basic than the same substituent in the para position. This is due to the steric hindrance created by ortho substituent, physically getting in the way of the lone pair.
(i) Effect of ortho substituent: For example, the electron-withdrawing group -NO2 in ortho nitro aniline, withdraws, electron density from the benzene ring which results in a decrease in electron density on N-atom. Hence, ortho nitro aniline has less basicity than aniline. The order of basicity is
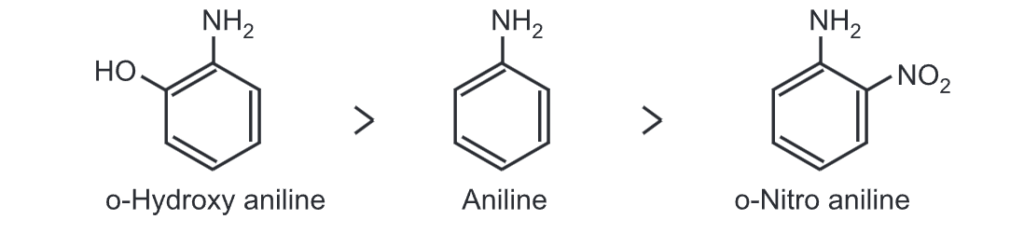
(ii) Stearic hindrance of substituents: Bulky substituents at ortho position in aniline may cause the loss of planarity of -NH2 group. It inhibits the delocalization of the lone pair of an electron from nitrogen to the benzene ring. It thus increases electron density on N-atom and hence basicity of amine.
(iii) H-bonding: The lone pair on N-atom may get involved in H-bonding with a hydrogen atom attached to the electronegative atom of the -ortho substituent present in the ring. It thus decreases electron density on N-atom and hence basicity of amine.
In methoxy anilines, the order of basicity is
p-methoxy aniline > m-methoxy aniline > o-methoxy aniline
the p-Methoxy group is increasing electron density on the ring by (+) resonance and keeps the nitrogen lone pair more localized on nitrogen. The m-methoxy group removes electron density from N-atom by (-) inductive effect, making it less basic than aniline. While the basicity of o-methoxy aniline is influenced by both resonance and inductive effects operating in opposite directions. In addition, H-bonding further decreases its basicity.
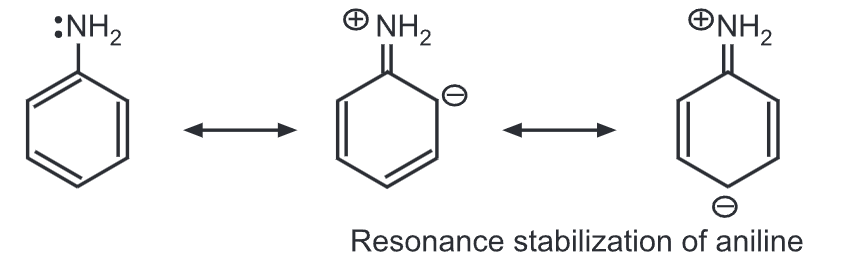
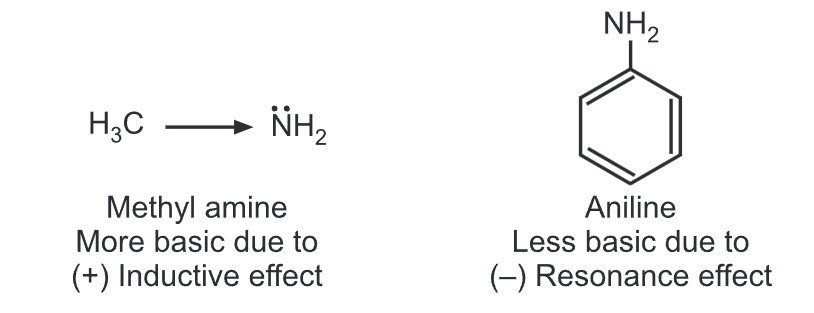
Aromatic amines are considerably weaker bases than aliphatic amines. Aniline is less basic than methylamine. In aniline, the lone pair of electrons on the nitrogen is partly engaged with the aromatic ring through resonance.
Hence, the electron density of nitrogen is reduced resulting in a less basic nature. While in methylamine, electron density on N-atom increases due to the electron-donating methyl group (i.e., (+) inductive effect).
In general, the order of basicity in amines is as follows:

Make sure you also check our other amazing Article on : Reaction of Amines