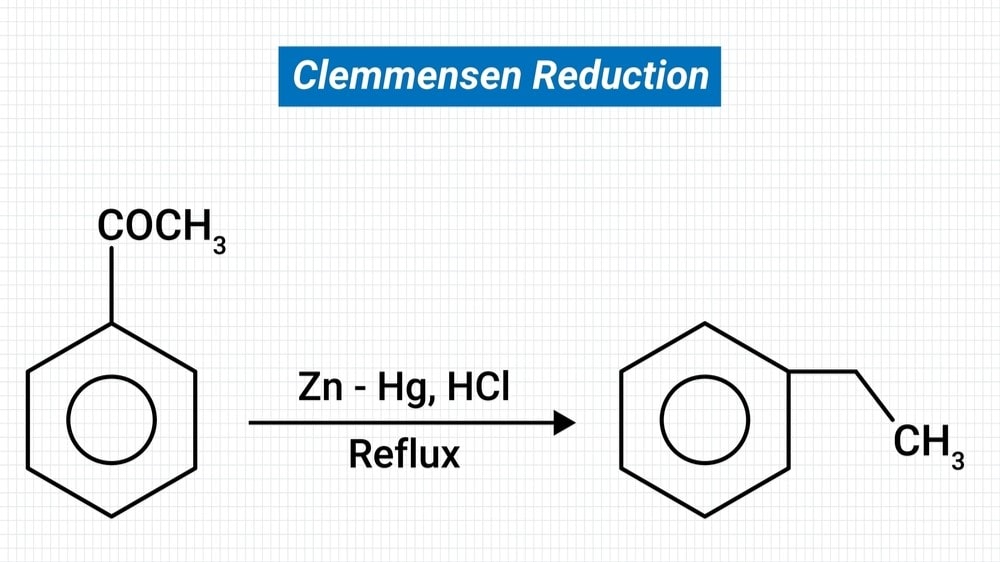
Definition: The conversion of a carbonyl group to a methylene group is done by Clemmensen reduction.
It consists of heating the aldehyde or ketone with zinc amalgam and aq HCl. Ketones are more preferentially reduced than aldehydes.
The reaction is highly specific for aldehydes and ketones and can be carried out with many other functional groups present.
Mechanism of Clemmensen reduction
The probable mechanism for Clemmensen reduction is
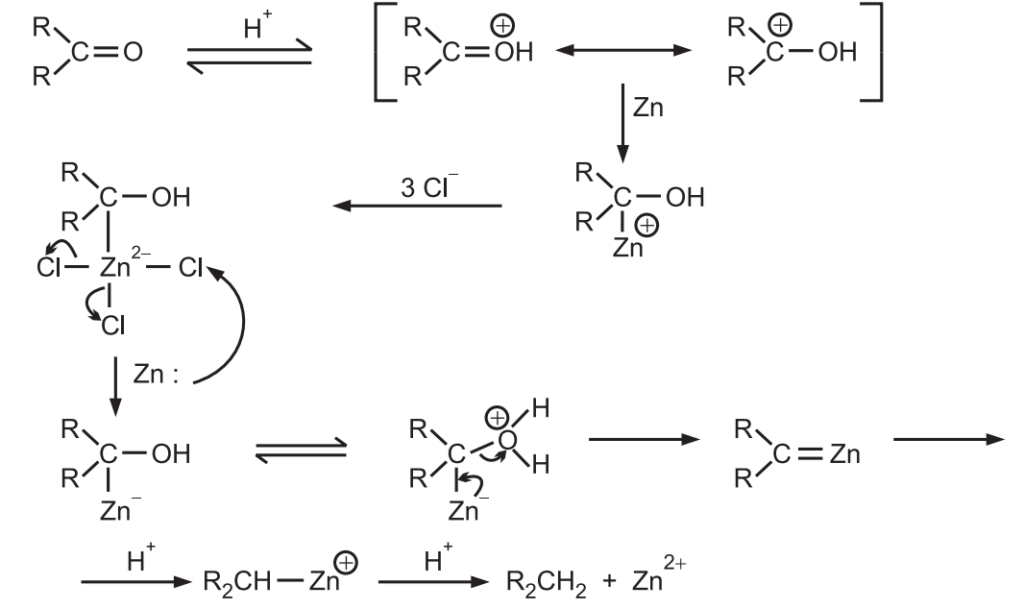
The concentrated acid is needed to force the initial protonation, amalgamation of zinc raises its hydrogen over voltage so that hydrogen is not produced.
Only haloacids are effective probably because by complexing the initial Zn species, they provide a medium for the reduction of this species by the second atom of Zn.
Drawbacks of Clemmensen Reduction:
- Purely aromatic ketones do not give satisfactory results. Pinacols and resinous products often predominate.
- The reduction of ketonic compounds of high molecular weight and very slight solubility is facilitated by the addition of a solvent such as EtOH, CH3COOH or dioxane which is miscible with aq. HCl.
- Keto acids in presence of EtOH give lower yields.
Meerwein – Pondorf Verley Reduction:
Definition: The conversion of a carbonyl compound into alcohol by the action of aluminium isopropoxide in an isopropyl alcohol medium is called the Meerwein-Pondorf-Verley Reduction.
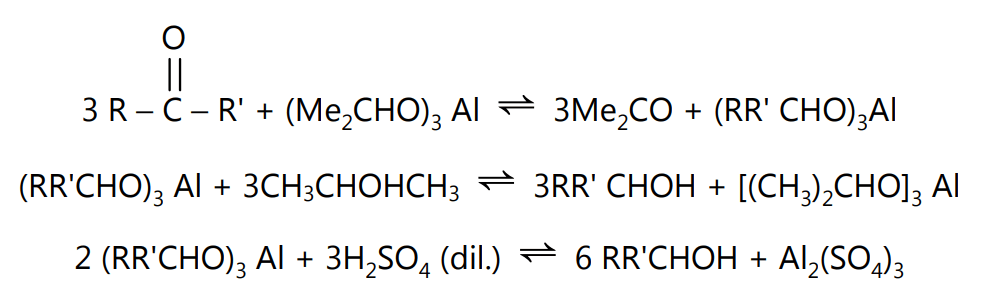
This is a reversible reaction. The equilibrium is shifted by the removal of the acetone by distillation.
The reaction takes place under very mild conditions and is highly specific for aldehydes and ketones so that C = C bonds and others like C + C, OR, − NO2, etc. do not get reduced by this reagent.
Mechanism: It involves the direct transfer of hydride from the α − C of aluminium isopropoxide to the carbonyl carbon reversibly.
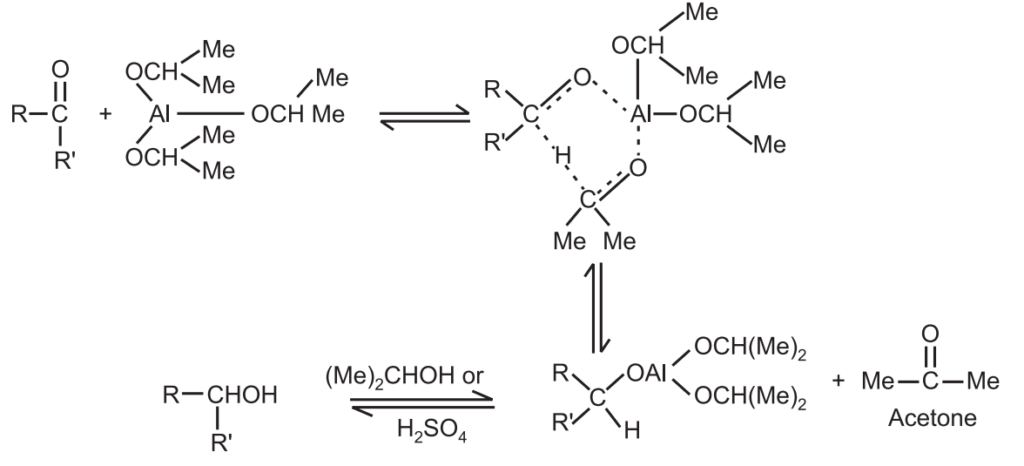
This is a three-step mechanism.
(1) Coordination of the ketone with the aluminium isopropoxide.
(2) The hydride transfer from the α − H of the aluminium isopropoxide to the carbonyl carbon atom of the carbonyl compound.
(3) Separation of the new complex.
Final Step: Generation of the alcohol from the computer
Stereochemistry
The product is the racemic [R/S] alcohol since the free energies of the two diastereoisomeric transition states, resulting from hydride attack on the si-face of the ketone or re-face, are identical.
Aluminium alkoxide derived from an optically pure secondary alcohol was used to effect a stereoselective reaction s − (+) − button−2−ol in the form of aluminium alkoxide, with 6-methyl heptane-2-one gave rise to two diastereoisomeric transition states which lead to an excess of s-6-methyl heptane-2-or derived from transition state (b) as expected from a consideration of the relative steric interactions.
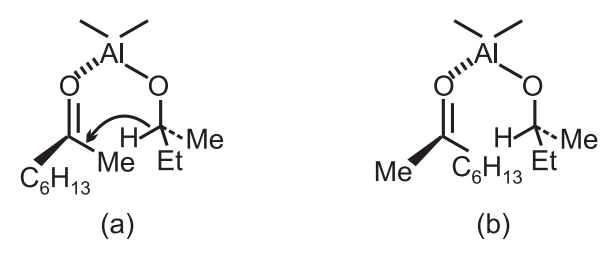
Transition state (a) has a less favourable Me − Me and Et − Hex interaction and hence requires higher free energy of activation; it, therefore, represents the less favourable reaction pathway.
Applications
Since the reduction is specific for the aldehydic and ketonic groups, the reaction is especially useful for the reduction of carbonyl compounds containing other reducible groups.
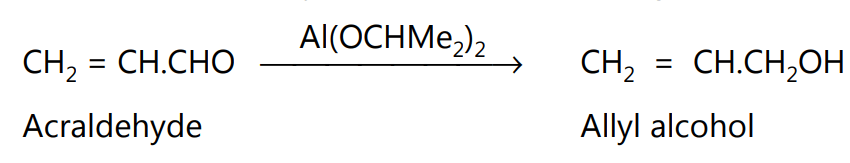
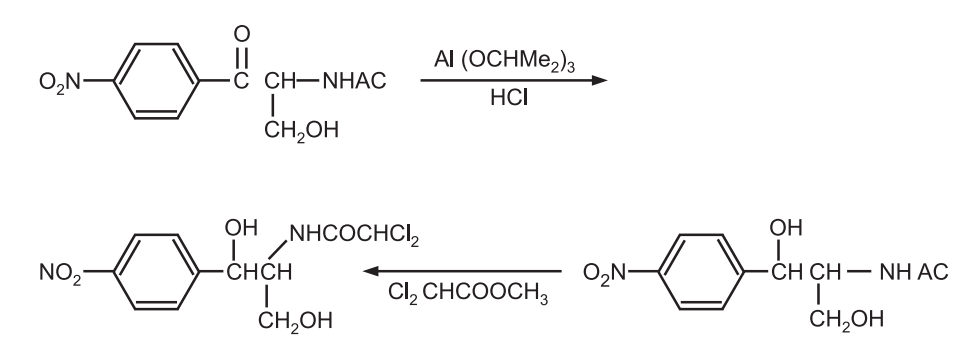
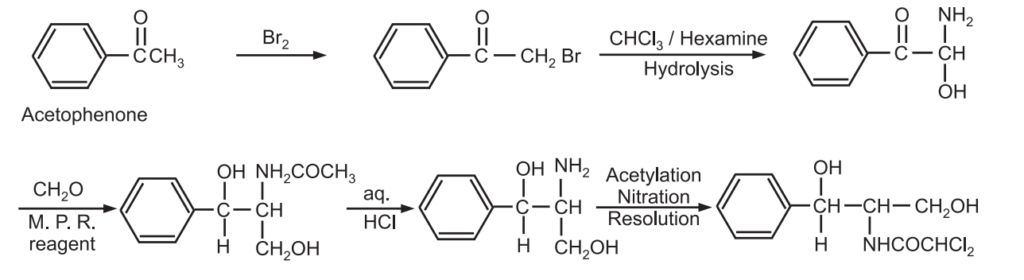
(a) Synthesis of chloromycetin
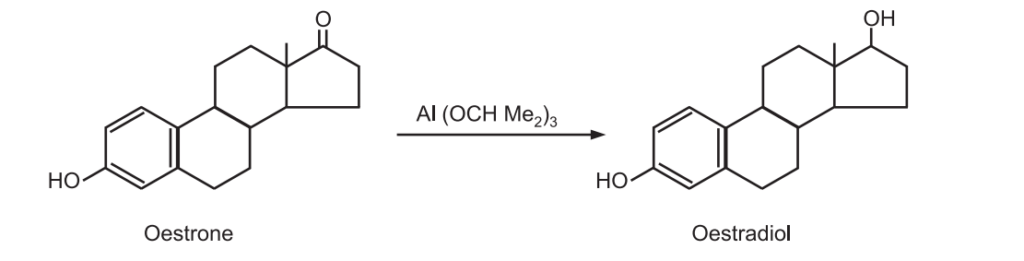
(b) Synthesis of oestradiol
(c) Synthesis of chloramphenicol
Advantages of Alisopropoxide over other Alkoxides:
Although a number of metals are used as their alkoxides for the reduction reaction, like Mg ethoxide, chloromagnesium ethoxide etc. the aluminium derivatives have been the best reagents. They are weaker condensing agents than Na or Mg alkoxides and are soluble in alcohol and hydrocarbon.
Applications
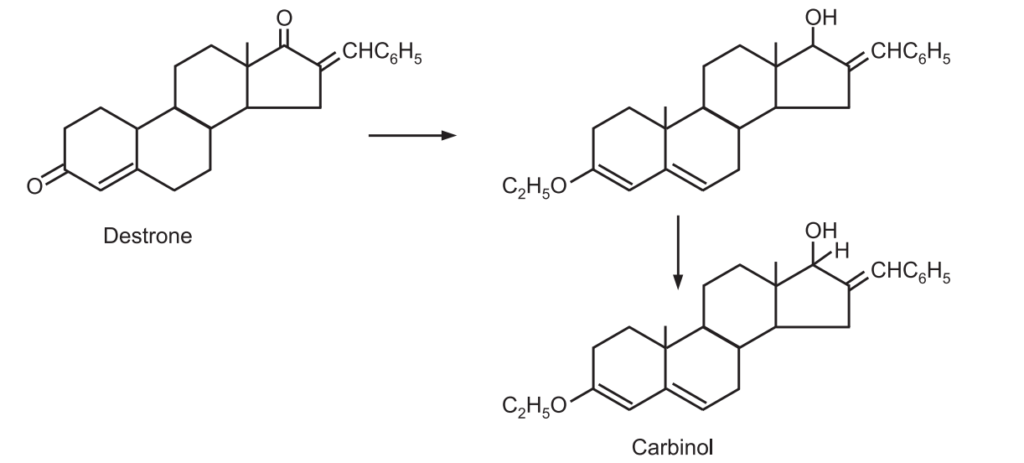
1. 16-benzalandrostenedione is reduced selectively to carbinol.
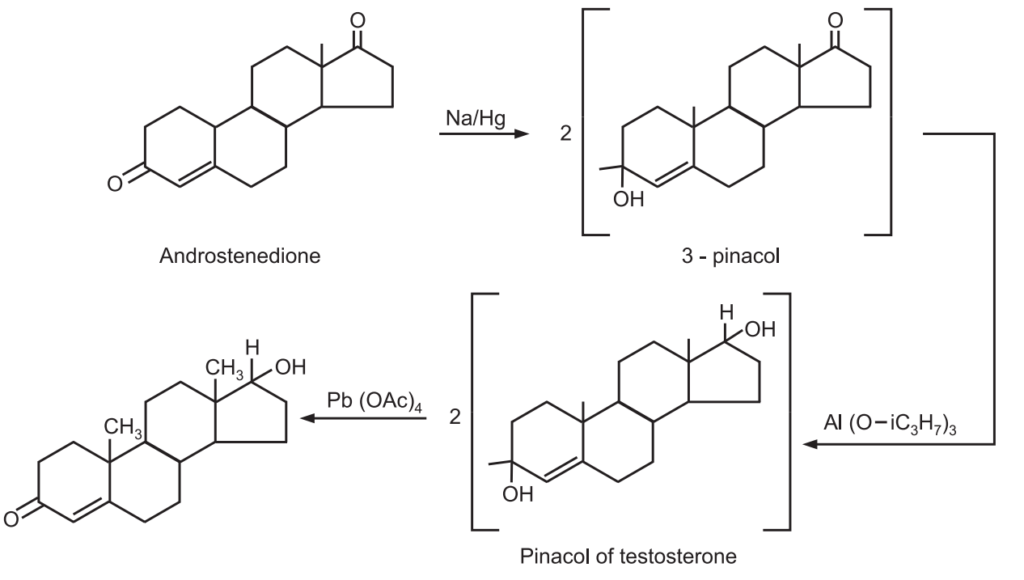
2. Androstenedione is converted into 3-pinacol with sodium amalgam and then reduced by aluminium isopro peroxide in 81% yield to the pinacol of testosterone. Treatment of the latter 1, 25 glycols with lead tetraacetate regenerated the ketone group with cleavage of the molecule to give testosterone.
Limitations:
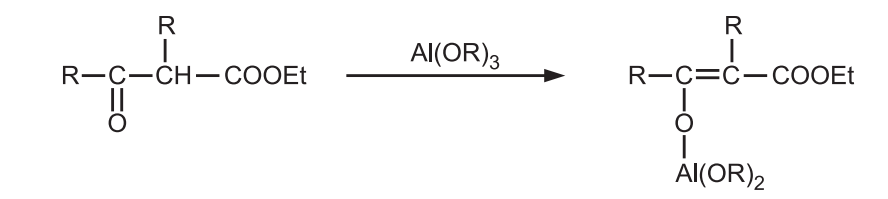
β-keto esters and β-diketones which are capable of enolization from the aluminium salt of the enolic form and are not reduced.
Make sure you also check our other amazing Article on : Birch Reduction