Aim: To determine the critical micelle concentration (CMC) of a surfactant (Sodium lauryl sulfate) by surface tension measurement.
Principles of Critical Micelle Concentration
The surface-active agents (surfactants) concentrate at the surface of a solution or at the interface between two immiscible liquids or between a liquid and a solid. They reduce the surface tension or interfacial tension.
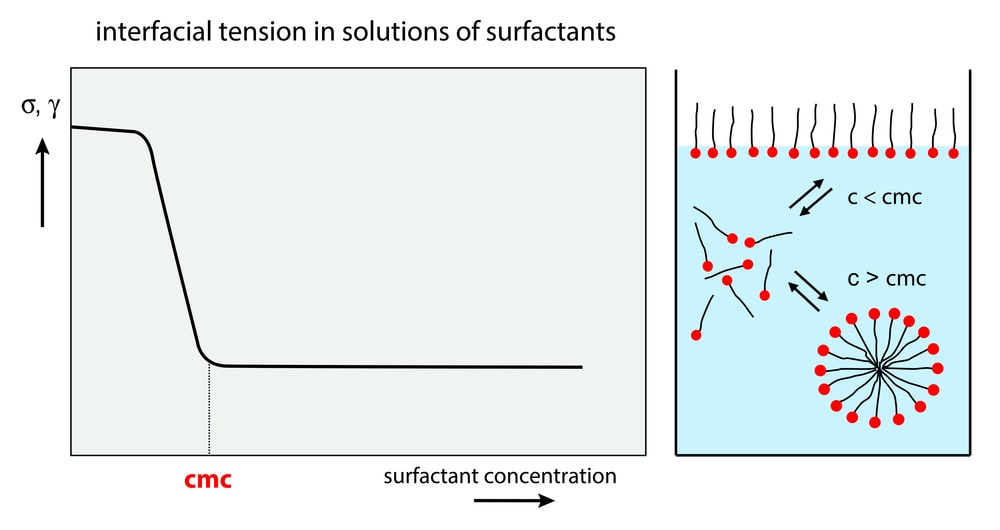
As the total concentration of surface-active agents in the aqueous phase is gradually increased, the concentration of surface-active agents undergoing adsorption at the surface is increased and this results in the gradual reduction of surface or interfacial tension. At one point both the surface and bulk of the aqueous phase become saturated with monomers of the surface-active agents. This concentration point is known as CMC. At this point, the surface or interfacial tension is reduced to the lowest value. Further increase in the concentration of surface active agent will result in aggregation of monomers forming micelle without any appreciable change in surface tension.
The surface tension Vs concentration curve for an aqueous solution of surfactant shows a progressive decrease in surface tension until the CMC is reached.
The CMC is taken at the point of interaction of the extrapolated straight lines on either side of the break in the curve.
Apparatus and Materials required: Stalagmometer, Pycnometer, Sodium lauryl sulfate (SLS).
Process:
- A stock solution of 5% sodium lauryl sulfate is prepared by dissolving 5 g of sodium lauryl sulfate in 100 ml water in a volumetric flask.
- A series of concentrations of surfactant (SLS) is prepared by diluting the stock solution: 50 mg/l00 ml (1ml to 100 ml), 100 mg/100 ml (2 ml to 100 ml), 150 mg/100 ml (3 ml to 100 ml), 200 mg/100 ml (4 ml to 100 ml), 200 mg /l00 ml (5 ml to 100 ml), 300 mg /l00 ml (6 ml to 100 ml), 400 mg/100 ml (8 ml to 100 ml) and 500 mg/100 ml (10 ml to 100 ml).
- The surface tension of the prepared solutions is determined by the drop count method.
- A graph of surface tension on the Y-axis and concentration on the X-axis is plotted.
Observation and Calculation:
Room Temperature: ____ 0C
| Sample | Concentration in mg/100 ml | Number of drops (mean of three observations) | Density | Surface tension |
| Water | ||||
The surface tension can be calculated for each concentration using the procedure described in the experiment “Determination of surface tension by drop counting method“.
CMC is determined from the surface tension Vs concentration curve.
Report: The CMC of SLS is ___________ mg/100 ml. (usually the concentration should be expressed in moles per liter. The concentration in mg/1000 ml should be divided by 1000 M where M is the molecular weight to get concentration in moles per liter.)
(The approximate value of CMC of SLS is 180 – 260 mg / 100 ml)
Make sure you also check our other amazing Article on: Determination of Surface Tension by Drop Count Method