Drug development process or life cycle
- Drug discovery (research)
- Drug development
- Registration
- Manufacture
- Marketing (post-marketing & pre-marketing)
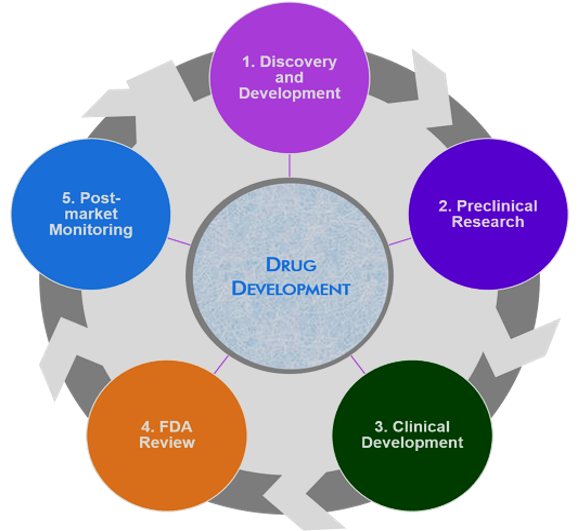
Table of Contents
Clinical Trial or Studies
Synonyms: Clinical trial, Clinical investigation, Clinical research, Clinical study
The branch of medical science deals with any research or study using living humans
Clinical trial: A clinical trial is the investigation of a drug in humans intended to discover or verify the effects of a drug, and to identify any adverse reactions with the object of ascertaining its safety and efficacy.
Types of clinical studies:
- Interventional studies (also called clinical trials)
- Observational studies
Interventional studies: Participants are assigned to groups & receive one or more interventions/treatments to evaluate the effects on biomedical or health-related outcomes. Participants may receive diagnostic, therapeutic, or other types of interventions.
Observational studies: Participants are identified as belonging to study groups and are assessed for biomedical or health outcomes. Participants may receive diagnostic, therapeutic, or other types of interventions, but the investigator does not assign participants to specific interventions/treatments.
Example: A patient registry is a type of observational study.
Investigational new drug: A new drug or biological drug that is used in a clinical trial but has not been approved by the FDA.
Investigator: An individual (researcher) who conducts a clinical trial (i.e., under whose immediate direction the drug is administered or dispensed to a subject). In the event, that an investigation is conducted by a team of individuals, the investigator is the responsible leader of the team.
Principal investigator (PI): The person who is responsible for the scientific and technical direction of the entire clinical study (for example, for all sites of a multisite study).
Subject: A human who participates in a clinical trial, either as a recipient of the investigational new drug or as a control. A subject may be a healthy human or a patient with a disease.
Sponsor (Lead): An organization person who takes responsibility and initiates a clinical investigation. He is responsible for analyzing the study data.
- An individual or
- Pharmaceutical company,
- Governmental agency,
- Academic institution,
- A private organization, or
- Other organization.
Sponsor-investigator: The person who both initiates and conducts the clinical study and under whose immediate direction the investigational drug is administered or dispensed.
Collaborator: A collaborator is an organization other than the Sponsor that provides support for a clinical study. This support may include funding, design, implementation, data analysis, or reporting.
Clinical trial protocol: The written description of a clinical study. It includes the study’s objectives, design, and methods. It may also include relevant scientific background and statistical information.
Responsible party: The Sponsor, Sponsor-Investigator, or Sponsor-designated Principal Investigator who is responsible for submitting information about a clinical study to ClinicalTrials.gov and updating that information.
Investigator’s Brochure (IB): It is a compilation of the clinical and non-clinical data on the investigational product(s) that are relevant to the study of the product(s) in human subjects.
Clinical Research Associate: The main function of a clinical research associate is to monitor clinical trials.
- He/she may work directly with the sponsor company of a clinical trial, as an independent freelancer, or for a Contract Research Organization (CRO).
- He/she ensures compliance with the clinical trial protocol, checks clinical site activities, makes on-site visits, reviews Case Report Forms (CRFs), and communicates with clinical research investigators.
Other Clinical Research Group
- Study Coordinator
- Data Manager /Biostatistician
- Regulatory Affairs Manager
- Clinical Trials Auditor
- Clinical Project Manager
- Clinical Research Manager
- Business Development Manager
- Drug Safety Associate
- Medical Writer Clinical Data Manager
Why a clinical trial?
- To discover or verify the effects of a drug
- To identify any adverse reactions to that investigational drug with the object of ascertaining its safety and /or efficacy.
Who Reads Clinical Trial Protocol?
- Investigators
- Physicians
- Nurses/CRAs
- IRB members
- Scientific reviewers
Clinical trials
- Carefully designed,
- Reviewed and completed,
- Approved before they can start
Medical interventions subjected to clinical studies
- Drugs,
- Cells and other biological products,
- Surgical procedures,
- Radiological procedures,
- Medical devices,
- Behavioral treatments and preventive care.
Who can participate in clinical trials?: People of all ages can take part in clinical trials, including children.
Age or age group: A type of eligibility criteria that indicates the age a person must be to participate in a clinical study. This may be indicated by a specific age or the following age groups:
The age groups are: Child (birth-17), Adult (18-64), Older Adult (65+)
Eligibility criteria: The key requirements that people who want to participate in a clinical study must meet or the characteristics they must have. Eligibility criteria consist of both inclusion criteria (which are required for a person to participate in the study) and exclusion criteria (which prevent a person from participating). Types of eligibility criteria include whether a study accepts healthy volunteers, has age or age group requirements, or is limited by sex.
Aim & Objectives of Clinical Trial
1. To raise the questions to be studied & clarify its importance
- Stage of development/phase of trial?
- Disease target?
- Subject population?
- Potential safety concern(s) in drug class?
- Sponsor?
2. To collect existing knowledge
3. To formulate hypothesis
4. To clarify ethical considerations
5. To achieve the objectives of the study: To find out whether a medical strategy, treatment, or device is safe and effective for humans to use or consume.
Potential Benefits of involving in clinical studies
- Provide the best approach for participants to play an active role in their health care.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
- Help others by contributing to medical research
CONTENTS OF CLINICAL TRIAL
1. Study summary
- Protocol title
- PI, and study coordinators
- Coinvestigators, sponsors
- Investigators affiliation
- Population
- Study sites and number of sites
2. General information about the study protocol
3. List of abbreviations
4. Objectives and rationale
5. Study design and methods
8. Inclusion and exclusion criteria
7. Informed consent form process
8. Adverse events reporting
9. Regulatory guidance
10. Assessment of safety and efficacy
11. Method of treatment of subjects
12. Ethical considerations
12. Data collection
13. Data access
14. Statistical methods
ClinicalTrials.gov: Database of privately and publicly funded clinical studies conducted around the world.
Phases of Clinical Trail
- Before pharmaceutical companies start clinical trials on a drug, they will also have conducted extensive preclinical studies.
- Clinical trials involving new drugs are commonly classified into five phases.
- Each phase of the drug approval process is treated as a separate clinical trial.
- The drug development process will normally proceed through all four phases over many years.
- If the drug successfully passes through phases 0, 1, 2, and 3, it will usually be approved by the national regulatory authority for use in the general population.
Phase 0: First in humans (FIH)
Phase 0 trials are the first in human trials. Single subtherapeutic doses of the study drug or treatment are given to a small number of subjects (10 to 15) to gather preliminary data on the agent’s pharmacodynamics (what the drug does to the body) and pharmacokinetics (what the body does to the drugs). For a test drug, the trial documents the ADME of the drug, and the drug’s interactions within the body, to confirm that these appear to be as expected.
Phase 1: Screening for safety.
Testing within a small group of people (20–80) to evaluate safety, determine safe dosage ranges, and begin to identify side effects. A drug’s side effects could be subtle or long-term, or may only happen with a few people, so phase 1 trials are not expected to identify all side effects.
Phase 2: Establishing the efficacy of the drug, usually against a placebo
Testing with a larger group of people (100–300) to determine efficacy and to further evaluate its safety. The gradual increase in test group size allows for the evocation of less-common side effects.
Phase 3: Final confirmation of safety and efficacy
Testing with large groups of people (1,000–3,000) to confirm its efficacy, evaluate its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow it to be used safely.
Phase 4: Safety studies during sales.
Post-marketing studies delineate additional information, including the treatment’s risks, benefits, and optimal use. As such, they are ongoing during the drug’s lifetime of active medical use. (Particularly relevant after approval under).
Make sure you also check our other amazing Article on: Classification Of Antianginal Drugs
