Aim: To study the effect of concentration on the rate of filtration.
Requirements: Chemicals: Calcium carbonates And water
Instruments/ Apparatus: Beakers, Buchner funnel Of Various sizes, Glass rod, Measuring Cylinder, Stopwatch.
Principle:
The rate of filtration depends on the concentration of solids. As the concentration of solids in the suspension increases the thickness of the filter cake increases. As a result, the rate of filtration decreases. This can be estimated by collecting a definite quantity of filtrate in the given interval for a given set of conditions.
Procedure:
(Effect of concentration on rate of filtration):
- Buchner funnels with filter paper are arranged properly.
- Different concentrations of 100 ml of calcium carbonate slurry (5%, 10%, and 15% w/v) are prepared in water
- The prepared slurries are passed through filtration assembly separately.
- The time required to collect 50ml of filtrate is recorded.
- The rate of filtration is calculated using the following equation.
Observation And calculations:
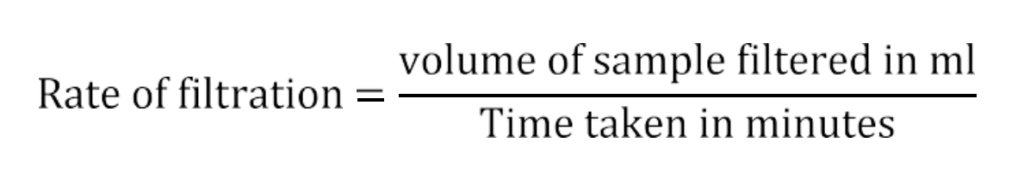
Observation Table:
| Sr. no | Sample Name | Concentration Of Solution | Filtration time required for 50 ml of filtrate (min) | Rate of filtration (ml /min) |
| i | 5 % w/v calcium carbonate slurry | 5% | ||
| ii | 10 % w/v calcium carbonate slurry | 10% | ||
| iii | 15 % w/v calcium carbonate slurry | 15% |
Results:
Make sure you also check our other amazing Article on: To study the effect of viscosity on the rate of filtration
