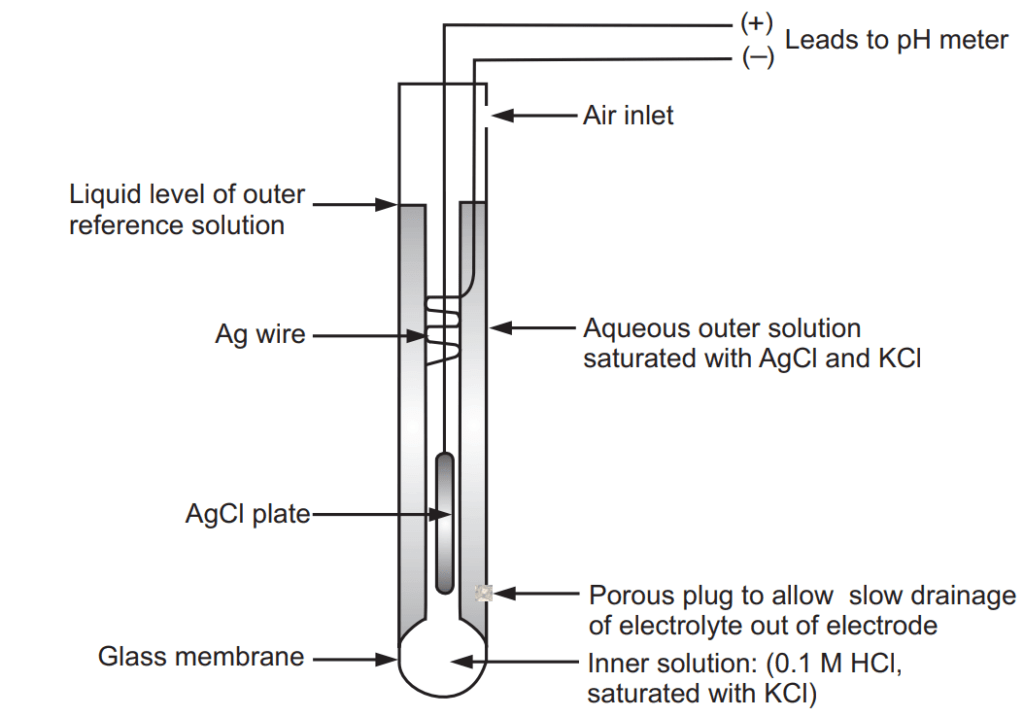
The pH of the sample is determined electrometrically using either a glass electrode in combination with a reference potential or a combination electrode. Electrometric pH determination is the measuring device is calibrated using a series of standard solutions of known pH. A pH is commonly measured with a potentiometric glass electrode. A pH electrode consists of two half-cells; an indicating electrode and a reference electrode. This may, however, be combined into a single probe, called a “combination pH electrode”. A pH electrode contains a bulb at the end covered with a thin glass membrane, Fig. 1.1. This membrane becomes hydrated in the presence of water. Hydrogen ions can enter the silicon-oxygen structure of the glass and alter the charge. This creates a change in electrical potential with respect to the silver/silver chloride reference. The free energy change is related to the change in hydrogen ion activity by equation (1).
G=-R ln [H+]1/ [H+]2 …………………(1)
where [H+ ]1 and [H+ ]2 are hydrogen ion activities of unknown and reference.
Glass pH electrodes respond to hydrogen ion activity rather than concentration. Activity may be expressed as the product of an activity coefficient (γ) and the hydrogen ion concentration.
[H+] = γ [H+]…………..(2)
where, the activity coefficient is a function of ionic strength and it has a value close to 1 in dilute solution. Glass electrodes respond to sodium ions to a slight extent causing errors under conditions of low hydrogen ion activity (i.e., high pH) and high sodium concentrations.
Table of Contents
pH Meter
The pH meter is a precise voltameter connected to the pH electrode which is very selective to ions. The pH meter can read small millivolt changes from the pH electrode system. The voltage produced by the pH electrode is proportional to the logarithm of the H+ activity. The pH meter display is scaled in such a way that the displayed results of measurement are just the pH of the solution.
pH measurement involves comparing the potential of solutions with unknown [H+] to a known reference potential. pH meters convert the voltage ratio between a reference half-cell and a sensing half-cell to pH values. The meter is seldom the source of problems for pH measurements. A successful pH reading is dependent upon all components of the system being operational. Problems with any one of the three: electrode, meter, or buffer yield poor readings. Over 90% of pH measurement problems are related to the improper use, storage, or selection of electrodes. Most applications today use a combination electrode with both half cells in one body. Today pH meters have temperature compensation (either automatic or manual) to correct for variations in slope caused by changes in temperature. Microprocessor technology has created many new convenience features for pH measurements such as auto buffer recognition, calculated slope, and % efficiency and log tables for the concentration of ions, etc.
In acidic or alkaline solutions, the voltage on the outer membrane surface changes proportionally to changes in [H+]. The pH meter detects the change in potential and determines [H+] of the unknown by the equation (3).
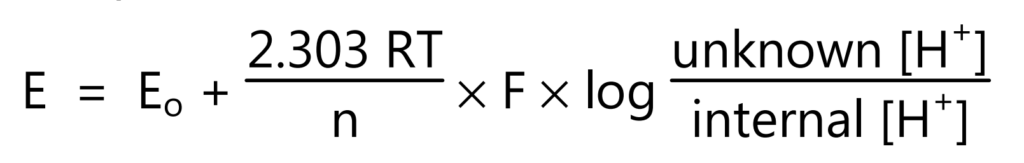
where E is total potential difference (measured in mV), Eo is reference potential, R is gas constant, T is the temperature in Kelvin, n is the number of electrons, F is Faraday’s constant, and [H+] is the hydrogen ion concentration.
Temperature Compensation
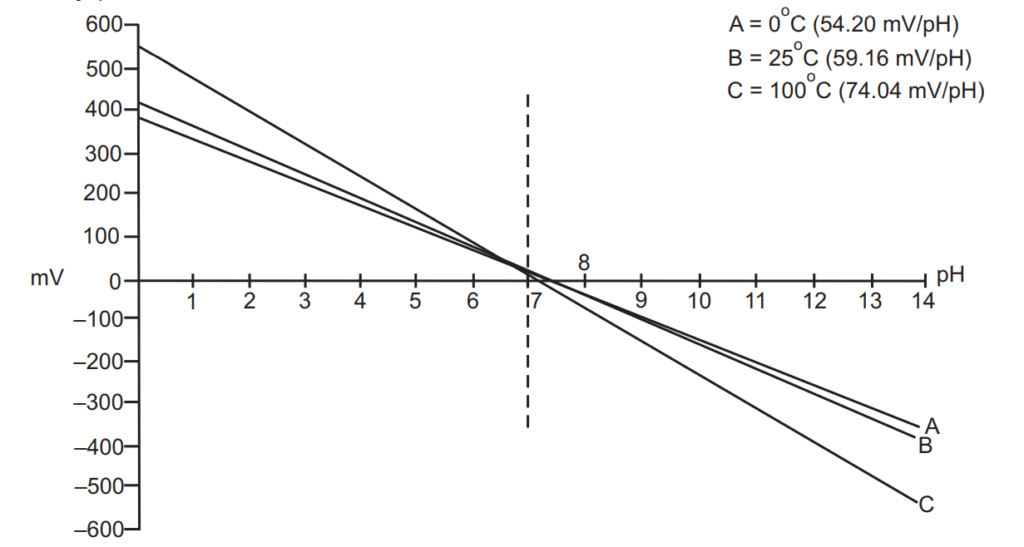
The pH of any solution is a function of its temperature. The voltage output from the electrode changes linearly in relationship to changes in pH, and the temperature of the solution determines the slope of the graph. One pH unit corresponds to the standard voltage of 59.16 mV at 25 °C and temperature to which all calibrations are referenced. The electrode voltage decreases to 54.20 mV/pH units at 0 °C and increases to 74.04 mV/pH units at 100 °C. Since pH values are temperature dependent, pH applications require some form of temperature compensation to ensure standardized pH values. Meters and controllers with automatic temperature compensation (ATC) receive a continuous signal from a temperature element and automatically correct the pH value based on the temperature of the solution. Manual temperature compensation requires the user to enter the temperature of the solution to correct pH readings for temperature is more practical for most pH applications. Although there are some restrictions on the use of the electrodes and the way they are treated between measurements, pH meters are in most cases the best way to check the pH of the solution, as they are much more precise than indicators and pH papers. Using a properly calibrated pH meter with a good electrode one may measure pH with ± 0.01-unit accuracy without any problem.
Ionization of compounds and hydrogen ion activity in the solution may be temperature-dependent. The actual pH of the sample changes with temperature due to a change in the hydrogen ion activity in the solution. Temperature compensation does not correct for this and is not desirable because accurate pH measurement is desired at that specific temperature. Temperature compensation only corrects for the change in the output of the electrode, and not for the change in the actual pH solution. Temperature also affects the glass membrane’s impedance (total effective resistance of an electric circuit). For each 8° below 25 °C, the specified impedance approximately doubles. Depending on the original impedance of the glass membrane, the meter must handle higher impedance at a lower temperature.
It is a fact that pH measurement determines only the concentration of active hydrogen ions in solution and is also responsible for the observed temperature dependence of measured pH values. For example, the pH of pure water at room temperature is 7.0. If the temperature increases, the dissociation of hydrogen and hydroxyl ions increases, and the pH decreases, even though the water is still charged neutral. Therefore, to predict the pH value of a solution at the desired temperature from a known pH reading at some other temperature, it is very important to know the relationship between the dissociation constant and temperature.
Colorimetric pH Determination
Colorimetric means to measure color. The colorimetric (photometric) pH determination method is based on the property of acid-base indicator dyes, which produce color depending on the pH of the sample. In the colorimetric method, chemicals are added to the water sample and those chemicals react with the water to produce a color change. The color indicates the pH of the water. The color can be measured visually or electronically as an absorbance change spectrophotometrically. The colorimetric method does not work when the water is already colored because it contains dissolved organic matter or large amounts of algae. Colorimetric test kits are inexpensive and can cover a wide range of pH values.
Application of Buffer
- Buffers are used in chemical analysis and calibration of the pH measurement system (an electrode and the meter). There can be small differences between the output of electrodes, as well as changes in the output over time. Therefore, the measurement system must be periodically calibrated. Most pH meters require calibration at several specific pH values. One calibration is usually performed near the isopotential point (the signal produced by an electrode at pH 7 is 0 mV at 25 °C), and a second is typically performed at either pH 4 or pH 10. It is best to select a buffer as close as possible to the actual pH value of the sample to be measured.
- Buffer’s resistance to changes in pH makes these solutions very useful for chemical manufacturing and essential for many biochemical processes. The ideal buffer for a pH has a pKa equal to the pH desired since a solution of this buffer would contain equal amounts of acid and base and be in the middle of the range of buffer capacity.
- Buffer solutions are necessary to keep the correct pH for enzymes in many organisms to live. Many enzymes work only under very precise conditions; if the pH is too far, the enzymes slow or stop working and can denature, thus permanently disabling its catalytic activity. A buffer of carbonic acid (H2CO3) and bicarbonate (HCO3− ) present in blood plasma, help to maintain a pH between 7.35 and 7.45. Pepsin is another example that shows maximum activity at pH 1.5.
- Industrially, buffer solutions are used in fermentation processes.
- Buffers can also be used to maintain the drug in its ionized as well as unionized form. The ionized form of a drug is more water-soluble than the unionized form. Buffers can be used to maintain a drug in its ionized (salt) form for aqueous solutions. The unionized form of a drug is more lipid-soluble than the ionized form. The unionized form, therefore, penetrates biological membranes much more efficiently than the ionized form.
- Amphoteric compounds are least soluble at isoelectric points. Substances such as proteins are purified based on this fact. Buffers are useful in maintaining the isoelectric pH. For example, insulin gets precipitated in the pH range of 5 to 6 and hence buffers are used for its purification.
- The pH can affect the stability of a drug in an aqueous solution. For example, ester drugs are very susceptible to hydrolytic reactions. Buffering formulations at low pH (pH 3-5) can reduce the rate of hydrolysis. Buffers also help to improve aspartame stability. Other examples are the alkaline instability of penicillin and ascorbic acid.
Make sure you also check our other amazing Article on : Sorensen’s Ph Scale