Pharmaceutical products contain a wide range of excipients in addition to active pharmaceutical ingredients for making the formula easy to manufacture, stable, effective, and convenient to the patient. These products in the pharmaceutical industry must meet high microbiological specs which means if they are not sterile, they should have not more than a minimal microbial population at the time of product release as well as afterward. If there is microbial spoilage of products then products are unsuitable for use, resulting in financial problems to the manufacturers and also a potential health hazard to the patients. Hence microbial spoilage is required to be minimized which depends on many factors affecting the microbial spoilage of pharmaceutical products that are enlisted as:
- Types and size of contaminant inoculum.
- Nutritional factors.
- Water.
- Storage temperature.
- pH.
- Oxidation-reduction balance (Redox potential).
- Package design.
Table of Contents
Types and Size of Contaminant Inoculum
Very low levels of contaminants which are unable to replicate in a product might not cause appreciable spoilage but, if a higher contaminant bio-burden occurs, the built-in protection could be insufficient and spoilage occurs.
This higher contamination arises if:
- Raw materials were unusually contaminated;
- A problem of the plant-cleaning protocol occurs;
- Biofilm detached itself from within supplying pipework;
- There was demolition or maintenance work in the vicinity of the manufacturing site
- Misuse of the product occurring during administration.
Nutritional Factors
Many spoilage microorganisms have simple nutritional requirements and metabolic adaptability which enables them to utilize many formulation components as substrates for biosynthesis, growth, and also trace materials contained in them. The use of animal products and crude vegetable materials in a formulation provides an additionally nutritious environment. Demineralized water prepared by ion-exchange methods, contains sufficient nutrients to allow significant growth of many water-borne Gram-negative bacteria like Pseudomonas spp.
Water
It is the most important cause of the survival and growth of micro-organisms. Some solute-rich medicines such as syrups appear to be ‘wet’, microbial growth in them may be difficult since the microbes have to compete for water molecules with the large numbers of sugar and other molecules of the formulation which also interact with water via hydrogen bonding. An estimate of the proportion of the non-complexed water in a formulation available to equilibrate with any microbial contaminants and facilitate growth can be obtained by measuring its water activity (Aw).
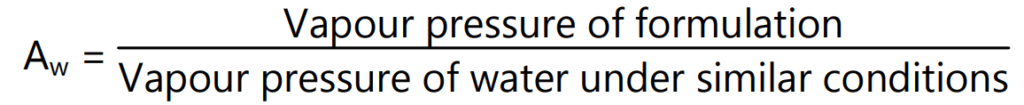
The greater the solute concentration, the lower is the water activity. Most microorganisms grow best in dilute solutions (high Aw) and, as solute concentration rises (lowering Aw), growth rates decline until a minimal, growth-inhibitory Aw is reached. The Aw of aqueous formulations is lowered to increase resistance to microbial attack by the addition of high concentrations of sugars or polyethylene glycols. Condensed water films are accumulated on the surface of ‘dry’ products such as tablets or bulk oils following storage in damp atmospheres with fluctuating temperatures, resulting in high localized Aw to initiate fungal growth.
Storage Temperature
The actual storage temperature determines the spoilage by particular types of microorganisms. Spoilage of pharmaceuticals occurs potentially over the range of about 20°C to 60°C. Storage in a deep freeze at −20°C or lower is used for long-term storage of foodstuffs and some pharmaceutical raw materials, and dispensed total parenteral nutrition feeds are stored in hospitals for short periods at −20°C to even further minimize the risk of growth of any contaminants that are introduced during their aseptic compounding. Reconstituted suspensions and multi-dose eye drop packs are sometimes dispensed with the instruction to ‘store in a cool place’ such as a domestic fridge (8°-12°C), or “store in the refrigerator “ partly to reduce the risk of in-use contamination growing before the expiry date. Water for Injections is recommended to be held at 80°C or above after distillation and prior to packing and sterilization to prevent possible regrowth of Gram-negative bacteria, and the release of endotoxins.
pH
Extremes of pH prevent the microbial attack. They grow at neutral pH, therefore acidic or alkaline formulations are less susceptible to spoilage. Around neutrality, bacterial spoilage is more likely, with reports of pseudomonas and related Gram-negative bacteria growing in antacid mixtures, flavored mouthwashes, and in distilled or demineralized water. Above pH 8, the spoilage is rare for soap-based emulsions. Products with low pH levels such as the fruit juice-flavored syrups are attacked by mold or yeast. Yeasts are metabolizing of organic acids and raise the pH to levels where secondary bacterial growth occurs. In the food industry, low pH adjustment is made to preserve foodstuffs only.
Redox Potential
The ability of microbes to grow in an environment is influenced by its oxidation-reduction balance since they require compatible terminal electron acceptors to permit the function of their respiratory pathways. The redox potential in the viscous emulsion is high due to the high solubility of oxygen in most fats and oils.
Packaging Design
It has a major influence on the microbial stability of some formulations in controlling the access of contaminants during both storage and use. The most important dosage form such as parenteral drugs is protected because of the high risks of infection by this route. Self-sealing rubber closures are used to prevent microbial entry into multi-dose injection containers following withdrawals with a hypodermic needle. Wide-mouthed cream jars are replaced with narrow nozzle and flexible screw-capped tubes to remove the likelihood of operator-introduced contamination during use. For medicines which rely on their low Aw to prevent spoilage, packaging such as strip foils is must be of water vapor-proof materials with fully efficient seals. Sacking, cardboard, card liners, corks, and paper are unsuitable for packaging pharmaceuticals because they are heavily contaminated with bacterial or fungal spores. These are replaced by non-biodegradable plastic materials. In the past, packaging in hospitals is frequently re-used for economic reasons.
Other factors affecting microbial spoilage of pharmaceutical products include:
- Relative Humidity
- Oxygen Availability
- Osmotic Pressure
- Surface Tension
Make sure you also check our other amazing Article on : Microbial Spoilage
