Good warehousing practices (GWP) mean storing supplies so that products are always available, accessible, and in good condition. Bad warehousing leads to damages resulting in losses. Pharmaceutical warehousing, therefore, is much more than the simple storage of products. It is an operation that preserves the integrity of drugs. According to cGMP Drugs must be stored to prevent contamination, and be positioned to allow for inspection and cleaning of the area. Each lot of drug product must be identified with a distinctive (and traceable) code, and the lot’s status must be identified (approved, quarantined, rejected). Written procedures must describe the distribution process for each drug. This includes procedures for recalls. Written procedures must describe the appropriate storage conditions for each drug. Different drugs can have vastly different requirements in terms of temperature, humidity, and lighting. The warehousing official ensures that the storage of each drug is in line with its specific requirements defined by the manufacturer. This can involve temperature-controlled warehousing and/or climate-controlled warehousing space20, both of which require state-of-the-art control and monitoring equipment to keep the space within specific environmental parameters.

Importance of Good warehousing practices
- To optimize the resources available for large-scale storage in a specified manner.
- As an integral part of the supply chain.
- Making the best use of the real-time data for effective supply chain and optimization of the stock put away and Bin utilization. To save time and effort in identifying and locating goods.
- To maintain a safe, clean and segregated environment.
- To control the movement and storage of material within the stores.
- To help in easy stock take and stock verification & reconciliation and help in stock corrections if necessary…
- The regulatory requirement for pharmaceuticals
- To develop a zone concept for product-wise segregation. To streamline the process of received, storage & distribution.
Good warehousing practices
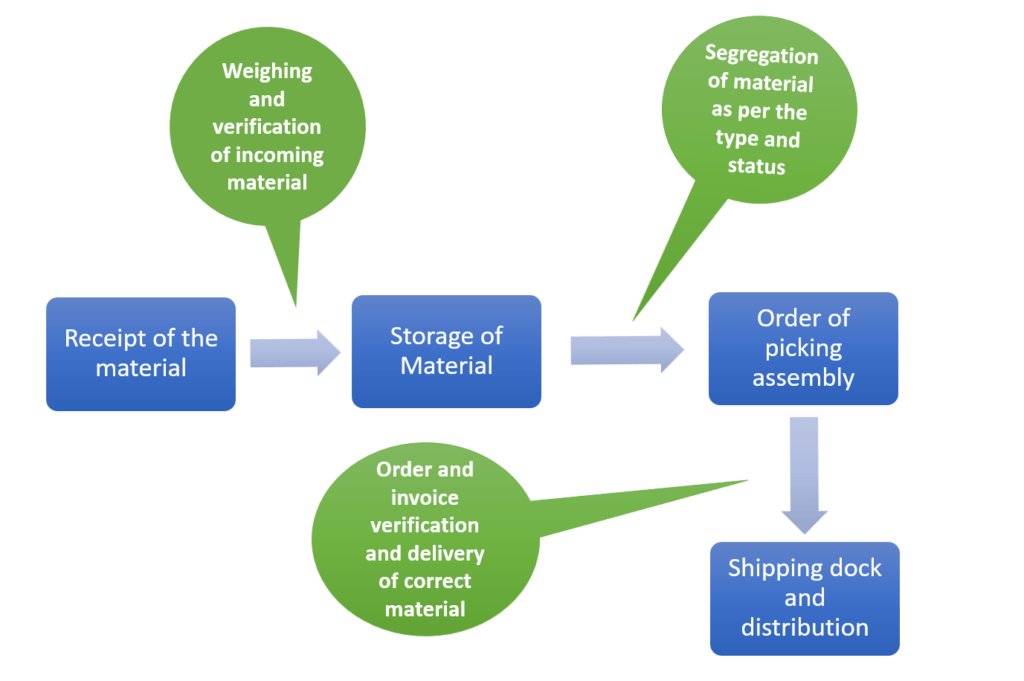
Warehousing & storage is an act of storage and assorting the finished goods so as to create maximum time utilization at the minimum cost. The key activities concerned with warehousing are.
- Receiving
- Identifying
- Holding
- Assembling and processing of the orders to meet the demand.
FUNCTIONS OF WAREHOUSING
Receiving & Recording of goods: While receiving the goods it is the responsibility of the warehouse dept to check and verify the goods that are coming into the warehouse by weighing the shipper coming in and counting the same. The correctness and quantity of the goods coming in should be verified at the time of receipt and recorded in a document. It should be mutually agreed and signed between the person transferring the goods and the person receiving the goods.
Storage: Major function of storage is to ensure that the product is protected and stored in a manner to ensure that the goods are easy to identify and as per the category. It is advisable to have a zoning concept where the products can be stored as per the zone.
Order picking: After the receipt of the order the line manager shall ensure that he has picked the same order as indicated in the packing list and the same batch number should appear in all the documents i.e. the invoice, the packing list, and the delivery note.
Distribution: The line managers hand over the goods to the packers who verify the goods against the delivery note and do the necessary marking on the shippers as per the customer.
- It is the responsibility of the loading supervisor to check the vehicle and confirm that it is matching as per the requirements and is clean and tidy for loading pharmaceutical goods.
- The incoming material in the warehouse is to be immediately sent to quarantine.
- The quarantine goods are to be sampled by the Quality Dept and sent for analysis… (In the case of a manufacturing unit the samples may be taken online before the batch is transferred to Quarantine).
- After getting released from the Quality Dept the goods need to be transferred to the Approved area.
- In case the goods are rejected they are supposed to be transferred to the Rejected area the keys to the rejected area shall remain with the Quality Dept.
- There should also be provided for customer returned goods and market returned Goods which should go through the Quality Dept for further segregation into either approved or rejected.
- Provision for goods to be stored under controlled temperature is a must
- This area should be mapped for temperature distribution.
- Another important area is for the controlled substances which can be misused and are required to be stored under BOND by the law.
Elements of Good warehousing practices
Costs involved in the warehouse: As a practice, it is good to follow the first expiry first out (FEFO) for the finished goods, which helps to maintain the inventory with the maximum shelf life. FIFO is also equally valid as FEFO. The imported goods need to be scrutinized and checked for expiry dates at the time of receipt.
Stock Verification: Orderly, timely, and frequent stock verification is the key to correct stocks, and the same affects the business positively. The warehouse must on a routine basis share the data on the non-moving, deadstock, and near expiry products so that the management can take a decision on the fate of the drugs. The data collected from the stock review should also be shared with the supply chain and planning Dept on a regular basis so as to facilitate an effective planning process.
Safety: Safety is of foremost importance in a warehouse considering the various types of activities and equipment like the forklift, Trolley, pallets drums shippers, etc. Some are kept at a height that if not stored properly can be precarious and lead to fatal accidents. OHSAS guidelines need to be followed religiously and all the employees should wear protective garments commonly called PPE22, to protect them from any accidental harm. Helmets, Safety shoes, garments, masks are necessary. Abrupt and rapid movements are uncalled for and cause more damage than benefits. So the warehouse employees need to be disciplined and cautious in their approach. Any and every accident should be reported immediately. The firing end emergency exit plans shall be well laid out and fire drills to be performed to validate the exit plan. The entire warehouse has to be subjected to pest control activities and the rodent baits should be checked at regular intervals. The baits” and chemicals should be kept away from the pharmaceutical preparations and at any given point should not come in contact with the workmen of the products kept in the area.
Premises, Health & Hygiene: The area should be kept clean and away from objectionable odors, smoke dust, and other contaminants. The warehouse should be well ventilated. It should protect the goods from adverse weather conditions. Opening leading to the entry of rodents, pests, birds, and vermin should be closed. Floors should be non-slip evenly graded to prevent stagnation and can be drained to trapped outlets protected by a grill The floor should be constructed using material that is impervious, non-toxic, non-absorbent, and crack resistant. Walls should be made of smooth, durable, impervious, non-adsorbent, and crack-resistant material that can be cleaned easily. All ceilings are to be constructed and finished so as to prevent condensation, leakage, and formation of molds and should be easily and s regularly cleaned. The door should be easily cleanable surfaces. Adequate lighting and lux levels. Toilets must not open directly into any place where the products are stored. The recommended storage conditions for cold storage are 2-8°C. which should be mapped and the temperature sensor to be placed at the hot spot identified manual temperature recording. Cold storage should not be overloaded should have racks inside for proper storage. It should be in sanitary condition at all times. The cleaning equipment should be placed in a well-designated area with proper labeling. Eating, drinking smoking, chewing gum or tobacco, littering, and undesirable behavior in the designated areas on the premises are prohibited. Any person who has open wounds and lesions boils sores or infectious disease must be sent on leave till they achieve complete recovery. Attested by a medical supervisor.
Good documentation: Last but not least is the documentation for the activities done…
- It’s a common saying in GMP that “if it is not documented it never happened”…
- SOP, records & Bin cards need to be checked, updated, and religiously followed.
- Maintain the invoices, delivery notes, and other documents.
Make sure you also check our other amazing Article on: Good Laboratory Practices
