Aim: To determine the buffer capacity of acetate buffer at various concentrations and find out the pH of maximum buffer capacity.
Principle of buffer capacity
The magnitude of resistance of a buffer to pH changes is called buffer capacity (\beta). The buffer capacity is defined as the ratio of the increment of a strong base (or acid) to a small change in pH brought about by this addition where is the small increment in gram \beta=\triangle B/\triangle pH equivalent per liter of strong base added to the buffer solution to produce a pH change of \triangle pH.
The buffer capacity is not a fixed value for a given buffer system but depends on the amount of base added.
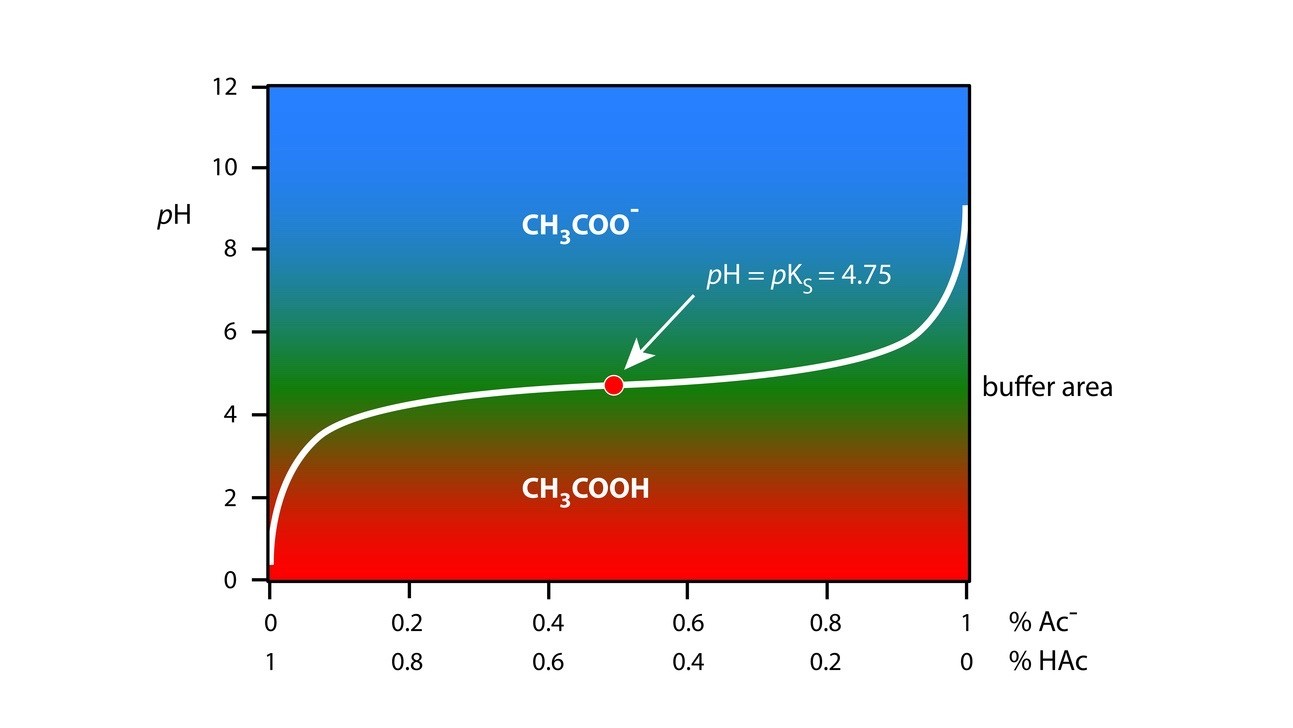
When sodium hydroxide is added to acetate buffer (acetic acid + sodium acetate), the concentration of sodium acetate, the salt term in the buffer equation, increases and the acetic acid concentration decreases proportionately. The changes in concentration of the salt and the acid by the addition of a base are represented in the buffer equation as:
pH=pK_a+\;Log\frac{\left[salt\right]+\left[base\right]}{\left[acid\right]-\left[base\right]}
when sodium hydroxide is added to a solution of acetic acid (excess), a mixture of acetic acid and sodium acetate forms. If sodium hydroxide addition is continued, there will be a change in the pH of the mixture, and buffer capacity at each point can be determined from the equation described above.
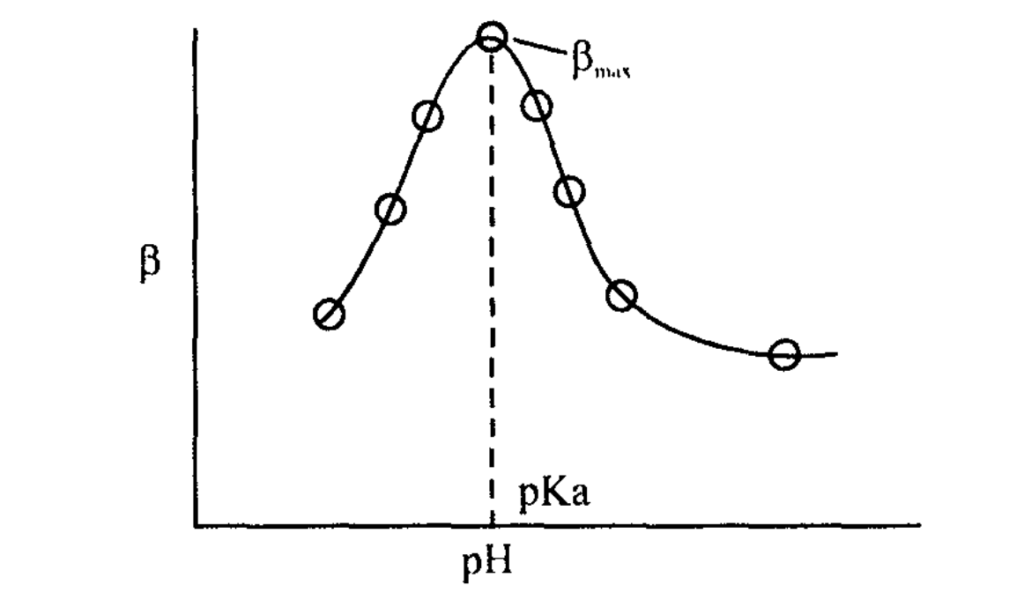
A plot of buffer capacity Vs pH will identify the pH at which buffer capacity is maximum.
Apparatus and materials required: Standard buffers, 0.4 M Acetic acid, 0.2 M Sodium hydroxide, pH meter, burette, and pipettes.
Process:
- The pH meter is calibrated using a standard buffer.
- 20 ml of 0.4 M acetic acid is taken in a beaker or conical flask and pH is recorded.
- 10 ml of 0.2 M sodium hydroxide is added to the acetic acid solution and the pH of the mixture is recorded after mixing. The process is repeated adding 2 ml 0.2 M sodium hydroxide every time till at least 30 ml sodium hydroxide is added.
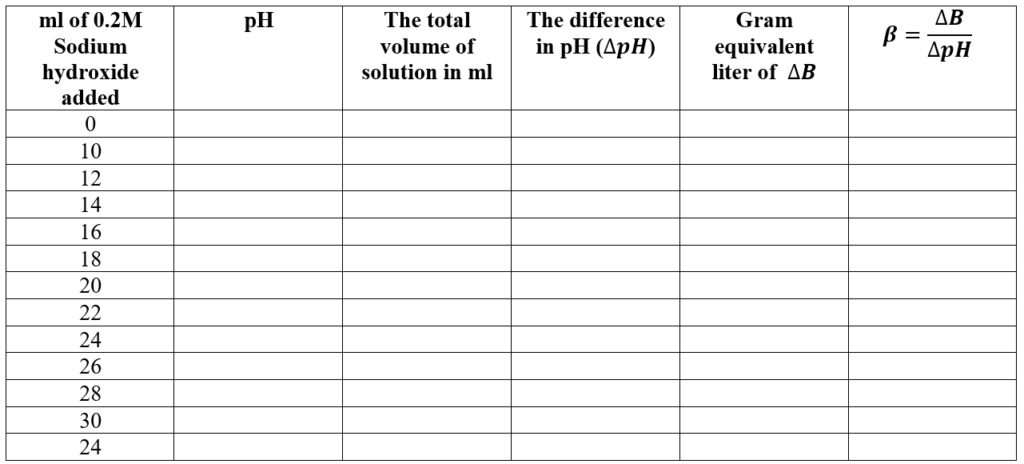
- Buffer capacity is calculated at each stage of addition.
Observation and Calculation:
20 ml of 0.4 M acetic acid solution is mixed with
The calculation for moles of sodium hydroxide added:
1000 ml of one Molar sodium hydroxide = 1 mol of sodium hydroxide. Therefore,
2\;ml\;of\;0.2\;M\;sodium\;hydroxide=\frac{1\times2\times0.2)}{1000} mol of sodium hydroxide.
When x ml of 0.2 M sodium hydroxide is added to a volume of 20 ml 0.4 M acetic acid, the total volume is (20 + x) ml; thus the concentration is \frac{1\times x\times0.2}{1000\times(20+x)}\; mol/ml.
Thus, , the molar concentration of sodium hydroxide after every 2 ml addition \frac{1\times x\times0.2}{1000\times(20+x)}\;=\;\frac{(x\times0.2)}{((20+x)\;)\;}\; mol/liter (M), where x is ml of 0.2 M sodium hydroxide added.
The pH at which the buffer capacity is maximum can be obtained from a plot of buffer capacity on the Y-axis and pH on X-axis as shown in the graph:
Report:
(i) The buffer capacity of acetate buffer:’
| Moles of sodium hydroxide added | Buffer capacity |
(ii) pH of maximum buffer capacity is ___________
Make sure you also check our other amazing Article on: Preparation of Buffer