Table of Contents
Prohibition of Import of Certain Drugs or Cosmetics
Following Drugs and cosmetics cannot be imported:
- Any drug or cosmetic which is not of standard quality;
- Any misbranded or spurious or adulterated drug or cosmetics;
- Any drug or cosmetic without an import license, for the import, for which an import license is prescribed.
- Any patent or proprietary medicine, which has not displayed the true formula or list of active ingredients with their quantities as per the label.
- Any drug which claims to cure or prevent any disease or ailments specified in Schedule J.
- Any cosmetic or drug-containing ingredient, which is unsafe or harmful.
- Any drug or cosmetic whose manufacture, sale, distribution, and import of which is prohibited by rule made under this act, except for the purpose of examination, test, or analysis.
- Drugs not labeled in the prescribed manner.
- Drugs after the expiry, and those which do not meet the standards, quality, and purity specified in the schedule-F.
Import of Drugs under License
The following classes of drugs can be imported under the license or permit granted by the licensing authority:
- Drugs specified in schedule C and C1 exclude those specified in Schedule X.
- Drugs specified in schedule X.
- Minor quantities of drugs are imported for examination, test, or analysis.
- Drugs for personal use are covered by a prescription of RMP.
- Any new drug.
- An application for an import License shall be made to the licensing authority by the manufacturer or by the manufacturer’s agent in India and shall be accompanied by a License fee of ` 1,000 for a single drug and ` 1,000 for each additional drug, duly signed by or on behalf of the manufacturer.
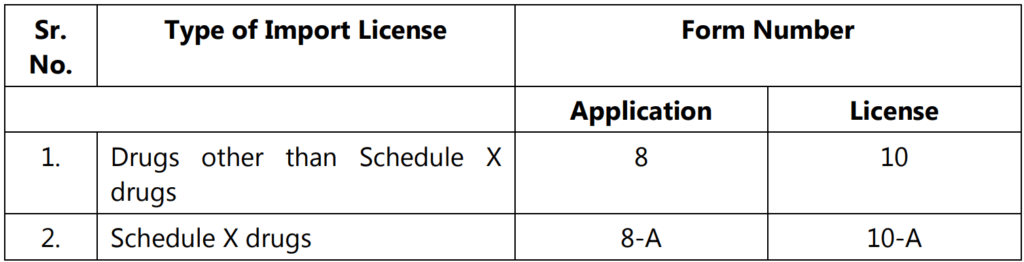
- Any application for import license in Form 8 or 8-A, shall be accompanied by a copy of the Registration Certificate issued in Form 41; in the case of emergencies, the issue of Import License by the central government in Form 10 or 10-A without the issuance of Registration Certificate under Rule 27-A, for reasons to be recorded in writing.
- The License remains valid up to 31st Dec of the year following the year in which its granted unless canceled or suspended earlier.
- The importer should have proper storage facilities for preserving imported drugs and properties.
- A fee of ` 250 shall be paid for a duplicate copy of the license, if the original is defaced, damaged, or lost.
Registration Certificate:
‘Registration certificate’ means, the certificate issued under Rule 27-A, by the licensing authority in Form-41, for the registration of premises and drugs manufactured by the manufacturer for import into and use in India.
- A fee of ` 1,000 and the US 500 dollars shall be paid through a Challan along with the application in Form 40 as a registration fee for his premises meant for manufacturing of drugs intended for import and use in India.
- A fee of US 300 dollars shall be paid for a duplicate copy of the Registration Certificate if the original is defaced, damaged.
Suspension and Cancellation:
Both the Import License and Registration Certificate will be suspended or canceled if the manufacturer or licensee fails to comply with any of the conditions. The licensing authority may after giving the manufacturer or licensee, an opportunity to show cause why such an order should not be passed, by an order in writing the reasons and further take measures for the same. The reasons for the cancellation may be:
- The drugs in schedules C and C1 are prohibited from being imported into the country after the expiry of the potency of the drug product.
- If the drug is banned in the country of origin then it is prohibited from importing into the country except for the purpose of examination, test, or analysis.
Conditions of Import License:
An Import License is subject to the following conditions:
- The licensee must observe at all the times the undertaking given by him or on his behalf in Form 9:
- The licensee must allow any authorized Inspector to:
- Enter the licensed premises where imported drugs are stored.
- Inspect the substances employed for testing.
- Take samples.
3. The licensee must furnish the adequate quantity of samples from the required batches to the licensing authority for examination along with complete protocols of the test applied.
4. If licensing authority so directs, until receipt of Certificate of Authorization, the licensee must not sell any batch products to which samples are submitted to the licensing authority.
5. The licensee must maintain the record of all sales of imported substances as prescribed under the rules and should furnish the same during the inspection.
6. The licensee must maintain separate records for the sale or distribution of Schedule X drugs.
7. Licensee must also comply with such further requirements, prescribed by the authority and of which he has been given not less than four months of notice.
Import of New Drugs:
- The written permission of the licensing authority is required for the import of new drugs.
- For obtaining permission, all documentary and other evidence related to the standards of quality, purity, strength, etc. should be supplied to the licensing authority.
- An application for an import License for small quantities of a new drug, as defined in rule 122-E for the purpose of treatment of the patient.
- Every application in Form 12-AA shall be accompanied by a fee of one hundred rupees for a single drug and an additional fee of fifty rupees for each additional drug.
- The fees shall be paid through a challan in the Bank of Baroda.
- A License for import of small quantities of a new drug, defined in rule 122-E, for the purpose may be canceled by the licensing authority for the conditions subject to which the License was issued. If so, the licensee may appeal to the Central Government within three months of the date of the order of cancellation.
Import of Drugs for Examination, Test, or Analysis:
- The drug is imported under a license in Form-11.
- The drug must be exclusively examined in the place specified in the license by the licensing authority.
- An authorized inspector must be allowed to investigate the manner in which imported substances are used; thereof allowed to take the samples.
- The record of the imported substances along with their quantities, the date of importation, and the name of the manufacturer should be maintained and reported to the authority.
- The licensee must comply with any further requirements as may be specified by the authority, and of which the licensing authority has given, to him not less than notice of a month.
- In case the license is canceled, the licensee may appeal to the Central Government within three months of the date of the order of cancellation.
Import of Drugs or Cosmetics for Personal use:
Import of drugs that are otherwise prohibited under section 10 of the act can be imported on following conditions:
- Drugs or cosmetics must be a part of a passenger’s bonafide baggage and must be intended for the exclusive personal use of the passenger.
- They must be declared to the customs collector, if so directed.
- The quantity of any single drug so imported must not exceed hundred average doses.
- Any drug or cosmetic not forming the part of passenger’s baggage may be allowed to import to an application made to the licensing authority in form 12-A.
- If the licensing authority is satisfied, a permit is granted in Form 12-B.
Note: Places through which Drugs may be imported into India:
- Ferozpur Cantonment and Amritsar Railway Stations: by rail (across the frontier with Pakistan).
- Ranaghat, Bangaon and Mohiassan Railway Stations: by rail (across the frontier with Bangladesh).
- Chennai, Calcutta, Mumbai, and Cochin: by sea
- Chennai, Calcutta, Mumbai, Delhi, and Ahmedabad: by air
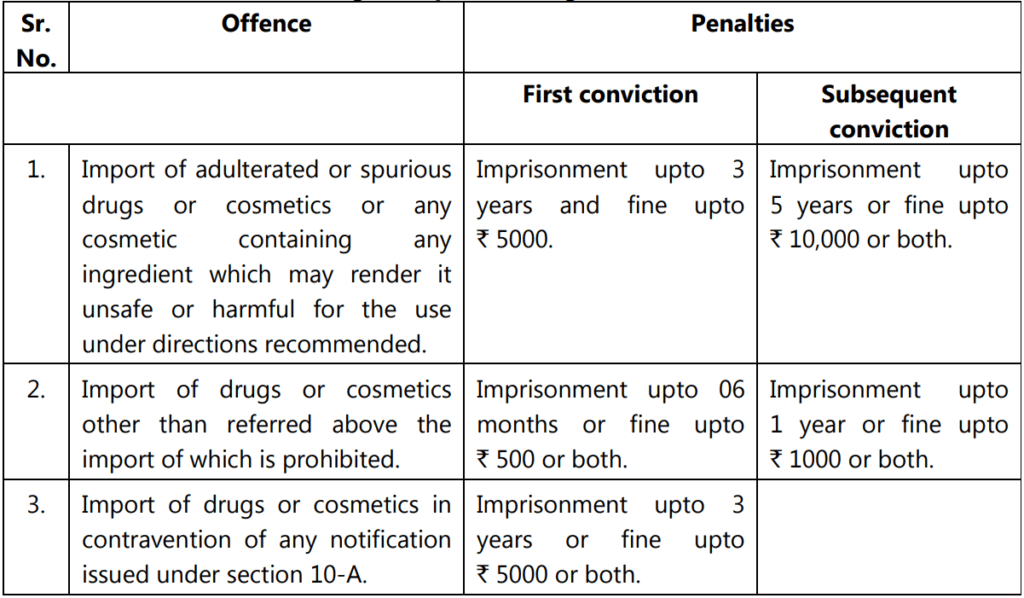
Offenses and Penalties Relating to the Import of Drugs:
Make sure you also check our other amazing Article on: Drugs And Cosmetics Act 1940
