Table of Contents
Prohibition of Manufacture and Sale of Certain Drugs
The following drugs are prohibited to manufacture for sale under section 18 of the act:
- Any drug or cosmetic which is not of standard quality or is misbranded, adulterated, or spurious;
- Any patent or proprietary medicine, whose formula with the quantities, is not disclosed on the label or container;
- Any drug which purports or claims to prevent, cure or mitigate any such disease specified in schedule J;
- Any cosmetic containing any ingredient which may render it unsafe or harmful for use;
- Any drug or cosmetic in contravention of this act or rules made thereunder;
Conditions for Grant of License
The license is granted, if the applicant complies with the following conditions:
- The manufacture must be conducted under active direction and personal supervision of competent technical staff (approved manufacturing chemist), as per the rules.
- The licensee and factory premises should comply with the conditions and requirements prescribed under Schedule M respectively.
- The applicant must provide for various operations, adequate space, plant, and equipment, as per Schedule M.
- The applicant must provide a separate testing unit or quality control section with an, independent head, with adequate facilities, for the test and standardization of drugs and raw materials.
- The applicant should make adequate arrangements for the storage of drugs, manufactured.
- For patent and proprietary medicines, the applicant must furnish the documents and data related to claims, safety, stability, therapeutic justifications, etc., as per the rules.
After completion of the inspection, Drug Inspector forwards a detailed report and his recommendations to the Central Licensing Authority.
- On receipt of application in the prescribed form along with fees for grant or renewal of license by the applicant, the authority verifies the statement, post-performance of the licensee, and the above requirements. Thereafter, the Licensing authority on necessary inquiries and satisfaction grants the license to the applicant in the prescribed form.
- If Licensing authority is of the opinion that the applicant is incapable to fulfill the requirements, it may refuse to grant or renew the license.
Types of Licenses for Manufacture of Drugs
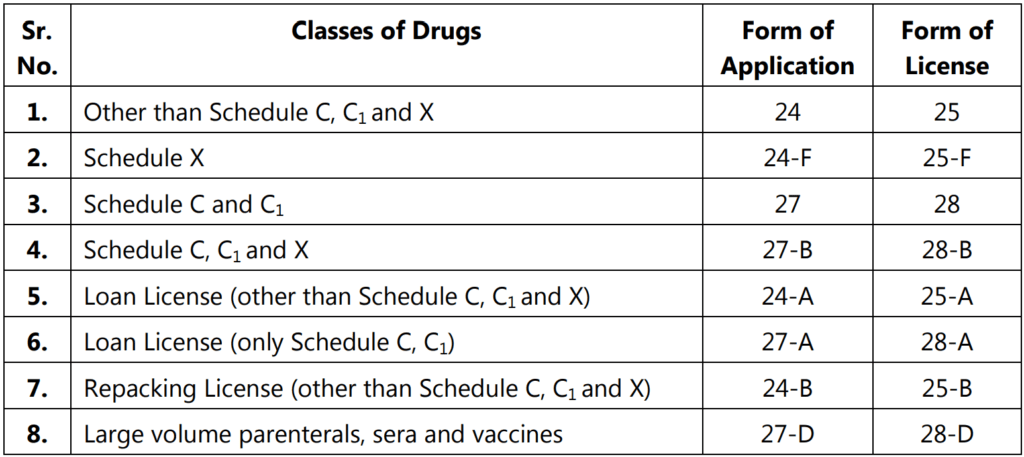
Conditions of License for Manufacture of Drugs
Manufacture of Schedule C, C1, and X Drugs:
Following are the general conditions of license of schedule C, C1, and X drugs in Form 28-B and 28-D; other than schedule C and C1 drugs in Form 25; Schedule X drugs Form 25-F.
Licensee must
- provide and maintain staff, premises, and equipment (as per Schedule M and Schedule M3 for medical devices).
- test raw materials and final products of each batch either in the laboratory approved by the licensing authority.
- maintain records of manufacture and testing of each batch as per schedule U.
- allow Drug Inspector to enter and inspect, premises, plant, the process of manufacture, means of standardization, and tests.
- allow Drug Inspector to inspect all the registers and records maintained under the rules and to take samples of manufactured drugs.
- provide the required information to Drug Inspector for ascertaining compliance with provisions of Act and Rules.
- time to time report to the licensing authority:
- Changes in expert staff responsible for manufacture or testing.
- Material alterations in premises or plant.
- samples of desired drugs and complete protocols of tests applied.
8. Do not sell any batch, the sample of which is submitted to the licensing authority, until receipt of Certificate of authorization is issued.
9. withdraw from sale remainder of any batch or recall drugs already issued, if licensing authority directs to do so.
10. Do not sell any drug manufactured under the license unless due precautions, necessary for preserving its properties, are taken throughout the period after manufacturing, also must maintain such quantities of reference samples.
11. comply with the provisions of Drugs and Cosmetics Act, 1940, rules thereunder and such further requirements from time to time published in Official Gazette.
12. maintain an “Inspection Book” in Form 35, to record impressions and defects noticed by Drug Inspectors.
13. comply with requirements of “Good Manufacturing Practices (GMP )” as per Schedule M.
14. The licensee has the license to manufacture schedule C, C1, and X drugs in Form 28-B must
- forward to the licensing authority every 3 months, a statement of sale to the manufacturers, wholesalers, retailers, hospitals, dispensaries, nursing homes, and registered medical practitioners.
- maintain as prescribed under rules, accounts of all transactions as regards to use, stock, manufacture, storage, and sale of schedule X drugs.
- the store always schedules X bulk drugs separately under the custody of a responsible person.
Manufacture of Drugs for Test, Examination, and Analysis:
A license is required to manufacture any drug in small quantity for the purpose of examination, test, or analysis purpose.
- If a person proposing to manufacture does not hold a license to manufacture drugs specified in Schedule C and C1 or other than Schedule C, C1, and X, shall obtain a license Form 29 before manufacturing such drugs.
- The licensee shall carry the manufacture and examination of drugs at the place specified in the license.
- In the case of drugs that are unsafe for use, a license in Form 29 can be granted only on producing NOC (no objection certificate) from the licensing authority.
- The application must be countersigned by the Head of the Institution, which proposes to undertake the manufacture.
- The license remains valid for a period of 1 year unless canceled or suspended.
- Any drug for the purpose of examination shall be placed in the containers, labeled for the purpose of manufacturing it, name and address of the manufacturer. Thereafter supplied to any other manufacturer, when necessary.
- The licensee shall allow Inspector to inspect the premises and satisfy himself that only examination is conducted.
- The licensee shall keep a record of the number of drugs supplied for analysis also maintain the ‘Inspection Book’.
- The licensee shall comply with such requirements specified and of which the authority has given him not less than 1 month’s notice.
Manufacture of New Drugs:
As per the Rule 122 E of the Drug and Cosmetics Rules 1945, a New Drug can be:
- A new substance of chemical, biological or biotechnological origin; in bulk or prepared dosage form; used for prevention, diagnosis, or treatment of disease in man or animal, which except during local clinical trials has not been used in the country; and which, except during local clinical trials, has not been recognized in the country as effective and safe.
- A drug already approved by the licensing authority for the proposed claims, which is now proposed to be marketed with modified or new claims; or A fixed-dose combination of two or more drugs, individually approved earlier for certain claims, which are now proposed to be combined for the first time in a fixed ratio; or
If the ratio of ingredients in an already marketed combination is proposed to be changed, with certain claims, like:
- Indications.
- Dosage form (including sustained release dosage form).
- Route of Administration.
- All vaccines shall be new drugs unless certified otherwise by the Licensing Authority.
- A new drug shall continue to be considered as a new drug for a period of four years from the date of its first approval or its inclusion in the Indian Pharmacopoeia, whichever is earlier.
Conditions of License:
- No ‘new drug’ can be manufactured, prior to approval from the licensing authority.
- The applicant shall submit data as given in Appendix-1 to Schedule Y, including the results of clinical trials as per the format of Appendix-2 to Schedule Y.
- While applying for the license, the applicant shall furnish the evidence certificate that the drug has already been approved.
Note: What is a Subsequent New Drug Application?
A Subsequent New Drug application is an application for approval of an already approved new drug by the Central Drugs Standard Control Organization (CDSCO). It can be made for the following cases:
- Bulk Drug already approved in the country (approved within 4 years).
- A new drug (Formulation) has already been approved in the country.
- A drug already approved and proposed to be marketed with a new indication.
- A drug already approved and proposed to be marketed as a ‘New Dosage Form / New Route of Administration.
- A drug already approved and proposed to be marketed as a ‘Modified release dosage form’.
- A drug already approved and proposed to be marketed with Additional Strength.
All the applications for approval of New Drug, Fixed-Dose Combination, and Subsequent New Drug are made under Form 44 (Application for grant of permission to import or manufacture a New Drug or to undertake clinical trial).
Make sure you also check our other amazing Article on: Import of Drugs
