Mentha pharmacognosy the Biological source of Mentha oil is obtained by steam distillation of flowering tops of Mentha piperita Linn.
Synonyms: Peppermint oil, Oleum mentha Piperita, Mint oil
Biological source: Mentha oil is obtained by steam distillation of flowering tops of Mentha piperita Linn.
Family: Labiatae.
Geographical source: It is cultivated in Japan, England, France, Italy, USA, Bulgaria, USSR and India (Jammu and Uttar Pradesh).
Cultivation and Collection:
The basic requirement for the cultivation of mentha is well-drained fertile sandy loam soil (pH = 7), rainfall (95 to 105 cm), temperature (15 to 25°C), and altitude (250 to 400 meters). The suckers are vegetatively propagated for cultivation purposes. The use of fungicides like mercury compounds enhances the better sprouting. The suckers are placed in the field in January or February month by keeping a distance of 10 cm. The distance between rows should be 50 to 60 cm. The foliar spray of urea and other fertilizers like superphosphate or potash is found advantageous. The harvesting should be done when plants reach the flowering stage. Thiodan (1: 700), endrine (1: 700), BHC (10 per cent), Sulphur (0.5 per cent) and malathion (5 per cent) are used for pest control.
Production of Mentha Oil:
The plants are dried in the air. Sun drying causes the loss of active principles i.e. volatile oils. The air-dried material is kept in an iron or steel vessel which has a false perforated bottom. The steam under pressure is passed through the drug for about 3 to 4 hours for complete distillation. Near about 80 per cent of oil is distilled in the first half of distillation. The medicinally important menthol is distilled in the latter half of distillation. The mentha oil is collected in a separating vessel where it is decanted and filtered. The average yield of oil is approximately 0.5 to 1 per cent v/w.

Morphological Characteristics:
Colour: Yellowish in colour or colourless
Odour: Agreeable, pleasant and characteristic
Taste: Aromatic, Pungent (followed by cooling sensation)
Solubility: Soluble in alcohol (70 per cent), alcohol, ether and chloroform insoluble in water.
Weight per ml: 0.9 to 0.912 gm.
Optical rotation: At 25°C −16° to −30°.
Refractive index: 1.4590 to 1.4650.
Chemical nature: Neutral to litmus paper
Chemical Constituents:
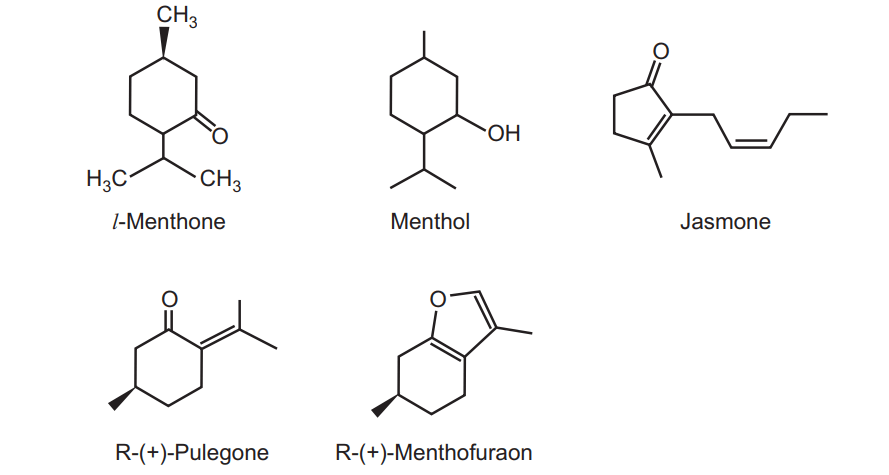
It mainly contains l-menthol (either in free form or ester form). There are three varieties of peppermint i.e. American variety (contains 80 per cent menthol), the Japanese variety (contains 70 to 90 per cent menthol) and the Indian variety which contains 70 per cent of menthol. Other important constituents are menthofuran, menthone, menthyl acetate, menthyl isovalerate, jasmone and other derivatives like cineole, l-limonene, isopulegone, camphene, pinene, jasmine and esters (possess pleasant odour). Menthofuran is responsible for bad odour due to resinification.
Chemical Test:
Take a few ml of oil and mix it with 5 ml nitric acid solution. The solution should be made by adding 1 ml of nitric acid to 300 ml of glacial acetic acid. Heat the solution in a water bath for 5 to 10 minutes. The liquid develops bluish colour which gets deep in colour upon more heating and shows copper-coloured fluorescence and after a few minutes, it turns golden yellow.
Uses of Mentha oil
The oil is used as a carminative, stimulant, flavouring agent and antiseptic. It is used in pharmaceutical preparations, cosmetic industries, toothpaste, tooth powder, shaving cream, chewing gum, candies, jellies, perfumery and essence manufacturing.
The other therapeutical uses of mentha oil are as spasmolytic, smooth muscle relaxant, digestant, anti-inflammatory, anti-ulcer, nasal decongestant, antitussive etc. It is used in the preparation of topical preparation and lozenges.
The oil should be stored in well-closed air tight umber coloured container because it becomes dark and viscous upon storage.
Make sure you also check our other amazing Article on: Liquorice Pharmacognosy
