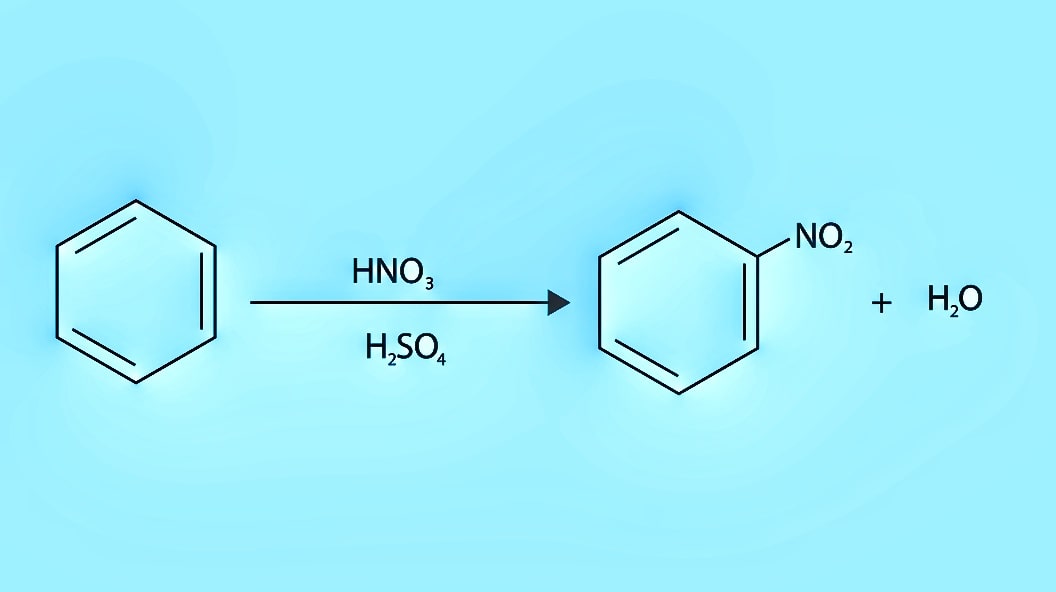
Nitration on Benzene: The most commonly used methods of nitration based on the order of their reactivity, include:
- a mixture of concentrated nitric acid and concentrated sulphuric acid.
- fuming nitric acid in acetic anhydride.
- concentrated nitric acid in glacial acetic acid.
- dilute nitric acid.
- nitrates of alkaline metals in the presence of sulphuric acid.
- nitrogen oxides.
- nitrates of metals in the presence of acetic anhydride and acetic acid.
- a mixture of nitric and sulphuric acids with glacial acetic acid or acetic anhydride.
- acetyl nitrate and benzoyl nitrate.
- nitrates.
(a) nitration of aromatic compounds by nitric acid involves the formation of water, i.e., a decrease in the concentration of nitric acid. Dilute nitric acid is a strong oxidizing agent, resulting in the formation of by-products. To avoid this, a nitrating mixture of concentrated nitric and sulphuric acids is usually used. The best yields of nitration products are obtained when 90% sulphuric acid is employed. The nitronium ion, NO; is generated in the mixture of concentrated HNO, and concentrated H2SO4 by the following reactions,
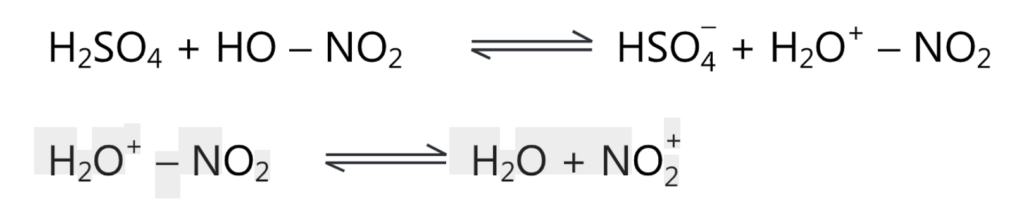
Nitric acid acts as a base and accepts a proton from H2SO4 to generate a nitronium ion. The addition of water lowers the nitrating power of this method by reducing the concentration of nitronium ions. Other strong acids like HCIO4, HF, and BF3 are also effective in place of H2SO4. Many salts such as NO2.CIO4, BF4, and CF3.SO; can bring about nitration. Because of their explosive nature, they are not normally used for nitration.
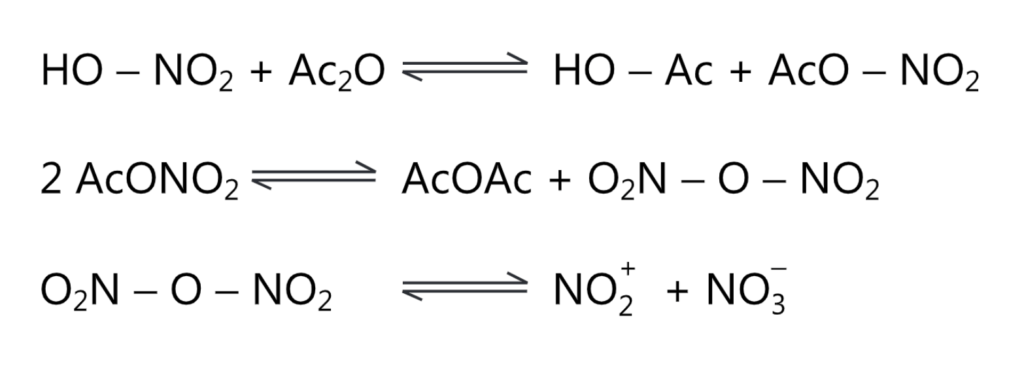
(b) A solution of nitric acid in acetic anhydride helps to generate nitronium ions by the following equation.
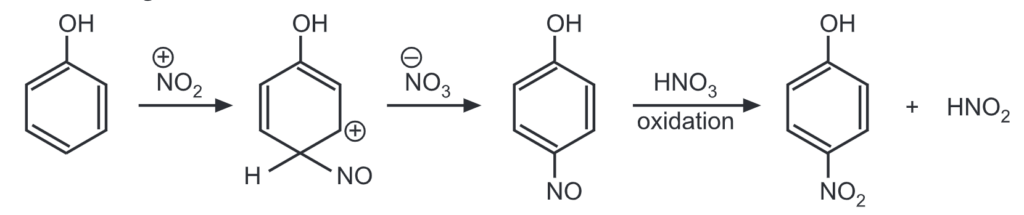
(c) Since, dilute nitric acid contains a very less concentration of nitronium ion, its nitrating ability is due to the presence of a small concentration of nitrous acid present in it. Nitrous acid generates nitrosonium ion, NO. The nitrosated ring then undergoes oxidation by nitric acid to give a nitro compound. This reaction liberates nitrous acid to continue the reaction.
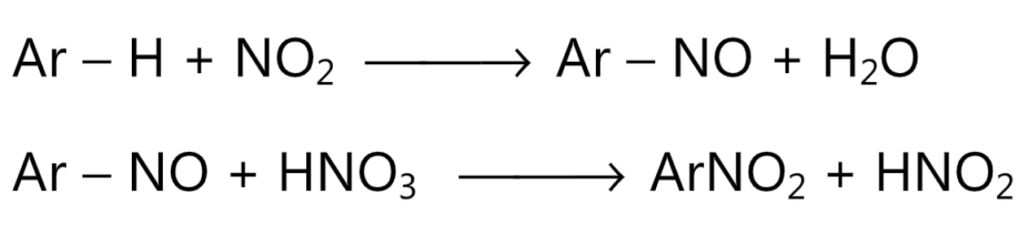
Hence, nitration on benzene this system can be used for nitration of only those compounds like phenols which can undergo nitrosation.
The general mechanisms by which nitration is found to occur are represented below:
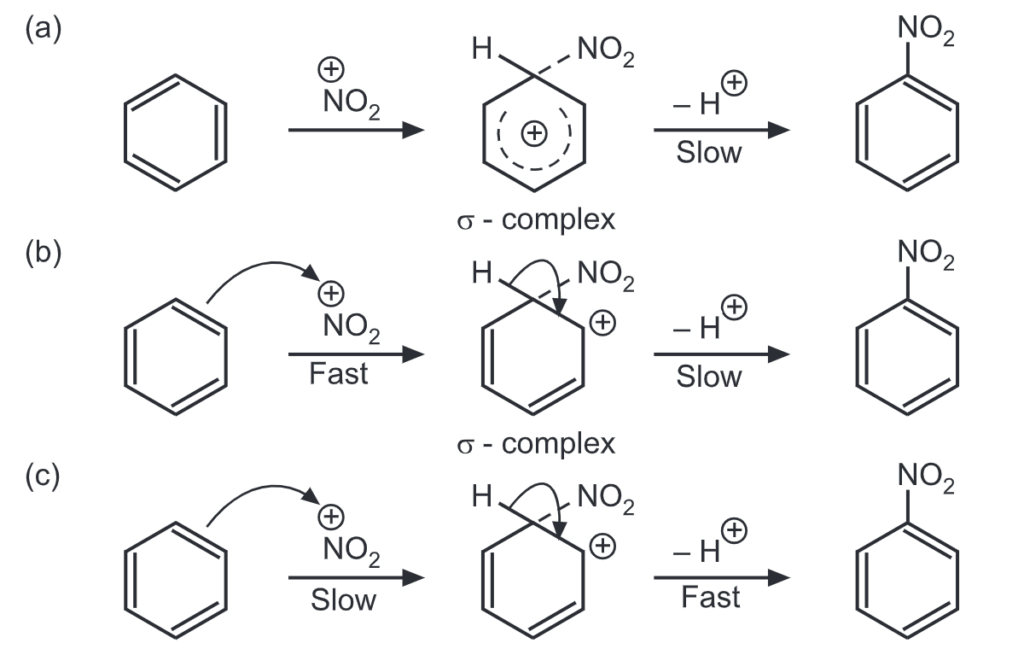
Temperature is a critical factor to govern monosubstitution. For example, benzene is converted smoothly into nitrobenzene at 50°-60°C. However, disubstitution is likely to occur if the temperature exceeds 60°C.
Phenol and toluene are nitrated more readily than benzene because they contain ortho para-directing substituents (-OH, -CH3), which make it easier for a nitro group to enter the ring. The substituents -CH3, -OCH3, -OC2H5, and -OH accelerate nitration in increasing order, while the substituents -COOH, -SO₂H, and -NO₂ retard it.
Make sure you also check our other amazing Article on : Electrophilic Substitution in Pyrrole