Plant tissue culture is described as the sterile and controlled cultivation of plant seeds, organs, explants, tissues, cells, or protoplasts on a chemically specified synthetic nutritional media under sterile and regulated light, temperature, and humidity conditions.
Table of Contents
The general procedure involves in plant tissue culture:
Sterilization of glassware and vessels:
All the required glassware is kept overnight dipped in sodium dichromate – sulphuric acid solution for removal of dirt, or any microorganisms, or any waxy materials. Then further cleaned with running tap water followed by double distilled water. Then placed inverted position in a plastic bucket for removal of extra water. Drying of glassware is carried out in a hot air oven at temp about 121o C for 30 minutes. For plastic materials, sterilization is carried out with non-abrasive detergent followed by cleaning under running tap water finally then rinsed with acetone for drying. All the cleaned materials are stored in a dust-proof cupboard for further use. All the stainless steel types of equipment, scalpels, forceps are sterilized with flame sterilization followed by wrapped with aluminum foil after cooling and kept in the dust-free cupboard. Other glasswares are also kept in sterile conditions by wrapped in aluminum foil with cotton pads for the prevention of dust particles.
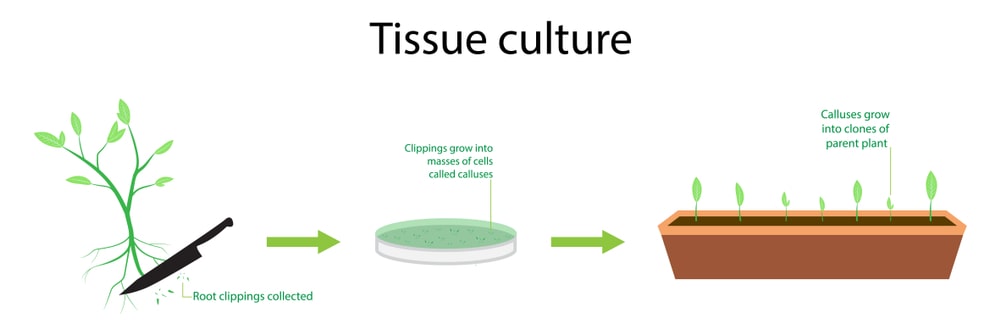
Preparation and sterilization of explants:
It is a very important step for plant tissue culture. Explants are selected as per the totipotency and are selected from any part of the plants such as young leaves, roots, flowers, stems, cambium, anthers, etc. After selection, the young part is removed with a sharp knife, and sterilization is carried out. Various types of sterilants are used for sterilization such as chromic acid (3-5 min), 0.1% mercuric chloride (3-10 min), 1-2% sodium or calcium hypochlorite (5-15 min), and 70% alcohol for 3 to 10 sec followed by washing with double distilled water. Generally, leaves are sterilized with 0.1% mercuric chloride solution followed by sterile water. Stems are sterilized with 2% sodium hypochlorite solution for 15-20 minutes followed by washing with sterile double distilled water. Seeds are sterilized with ethanol for 10 seconds followed by rinsing with purified water and then 10% calcium hypochlorite solution for 15 minutes. Fruits are cleaned with ethanol followed by 2% sodium hypochlorite solution for 10 minutes and finally sterile double distilled water washing for 15 minutes.
Production of callus from explants:
Sterile explants were further transferred aseptically into the sterilized medium and incubated into a BOD incubator for necessary growth. Temperature is maintained at 25°C ± 2°C kept for 2-3 weeks.
Proliferation:
Developed callus further cut into small pieces with sterile scalpel and transferred into another fresh medium for further proliferation. This process is also known as sub-culturing. This method is carried out at an interval of 4-5 weeks based on the growth of the callus.
Suspension culture:
It contains a uniform suspension of separate cells in a liquid medium. For this callus is transferred into the liquid medium and agitated continuously (80-150 rpm) in a BOD incubator for cells to separate. After cell growth, again sub-culturing is carried out.
Make sure you also check our other amazing Article on : Growth pattern