Pyrrole chemical reactions: Pyrrole, furan, and thiophene, all are π – excessive rings. Hence, all these rings are prone to electrophilic substitution reactions on the ring carbons. The order of reactivity for electrophile attack is pyrrole > furan > thiophene > benzene. The greater reactivity of pyrrole towards electrophiles is due to the greater electron releasing ability of the N – atom than oxygen and a sulfur atom. Thiophene is the least reactive.
These heterocycles are π – excessive rings. Hence, these heterocycles are less reactive toward nucleophilic substitution reactions except for deprotonation at the N-atom or C-atom. Pyrrole behaves both as a weak base and a weak acid. e.g.,
Hence, the proton attached to the nitrogen atom undergoes easy deprotonation in both acid and in alkali. Similar deprotonation of carbon atoms in the ring occurs only in more acidic conditions. The α – protons (C2 & C5) exchange occurs at twice the rate of the β-protons (C3 & C4).
Pyrrole Chemical Reactions
1. Alkylation and arylation: The sodium/potassium salt of pyrrole reacts with an alkyl halide to give corresponding N – alkyl pyrrole. The presence of electron-withdrawing substituent on the pyrrole ring favors rapid N-alkylation or N-arylation.
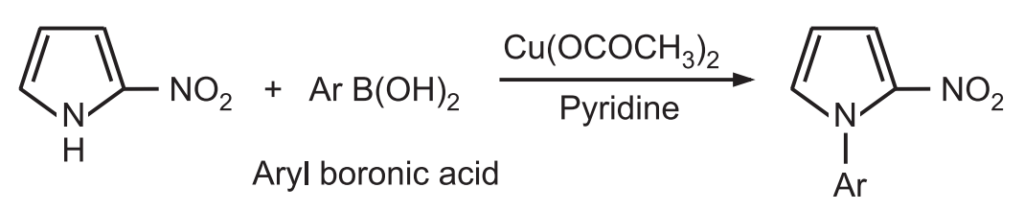
However mono C – alkylation of pyrrole can not be achieved by direct reaction with alkyl halides.
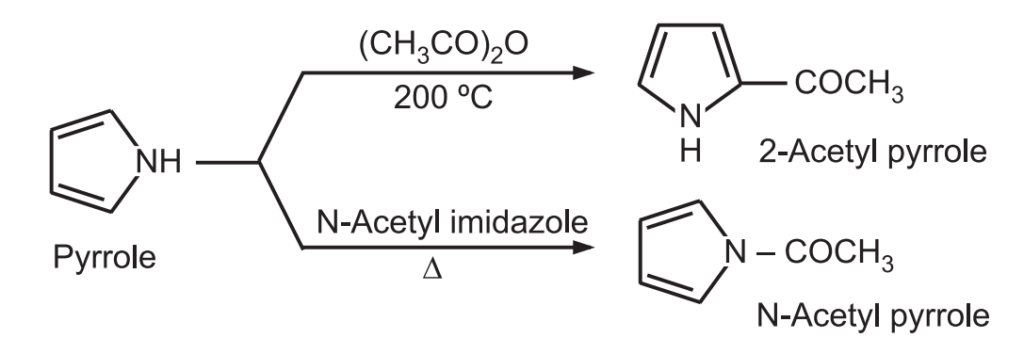
2. Acylation: Pyrrole treated with acetic anhydride at 200°C gives 2 – acetyl pyrrole while N – acetyl pyrrole can be obtained by heating pyrrole with N – acetyl imidazole.
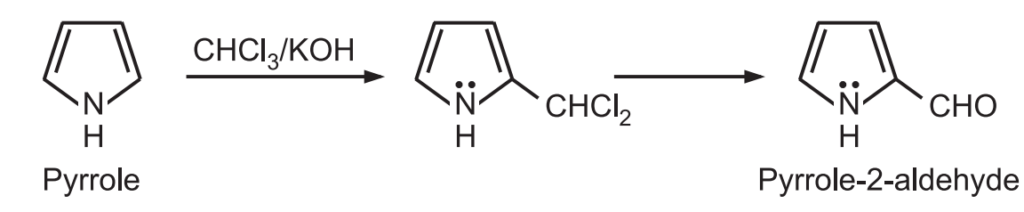
3. Reimer – Tiemann reaction: In the presence of a strong base and chloroform, pyrrole undergoes Reimer – Tiemann reaction to form pyrrole – 2 – aldehyde.
4. Ring expansion: When potassium pyrrole is heated with chloroform and sodium ethoxide, the pyrrole (five-membered ring) expands to pyridine (six-membered ring).
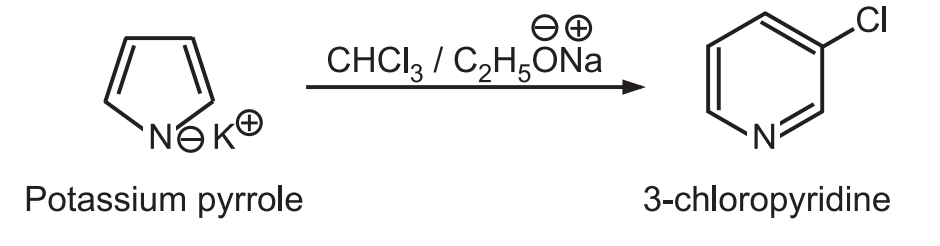
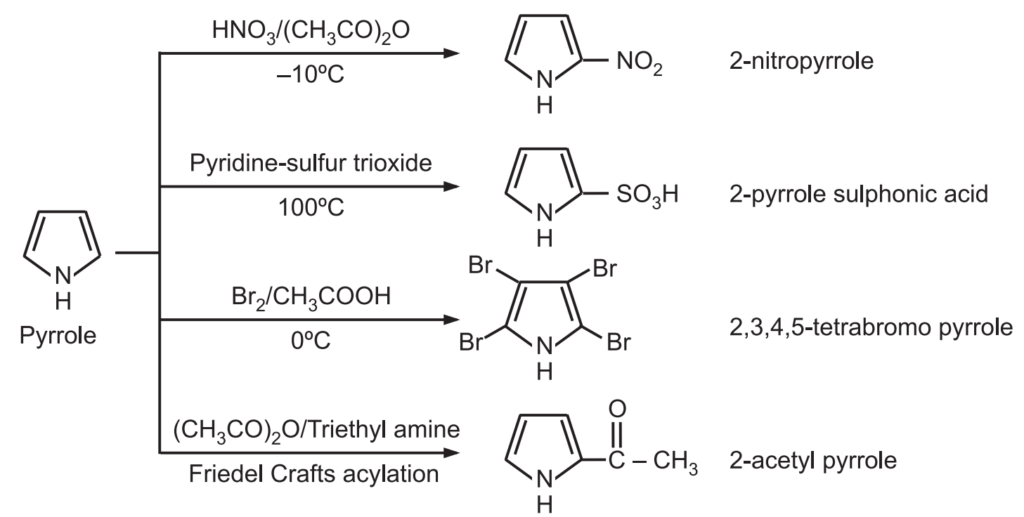
5. Electrophilic substitution: The electrophilic substitution takes place preferably at C2 or C5 – positions. If these positions are already occupied then substitution takes place at C3 or C4 positions.
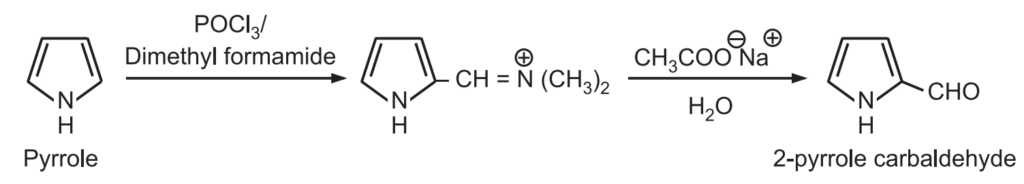
6. Vilsmeier-Haack reaction: Pyrrole may be formylated by heating it with phosphorus oxychloride and dimethyl formamide. The intermediate is hydrolyzed in the presence of a mild base to 2 – pyrrole carbaldehyde.
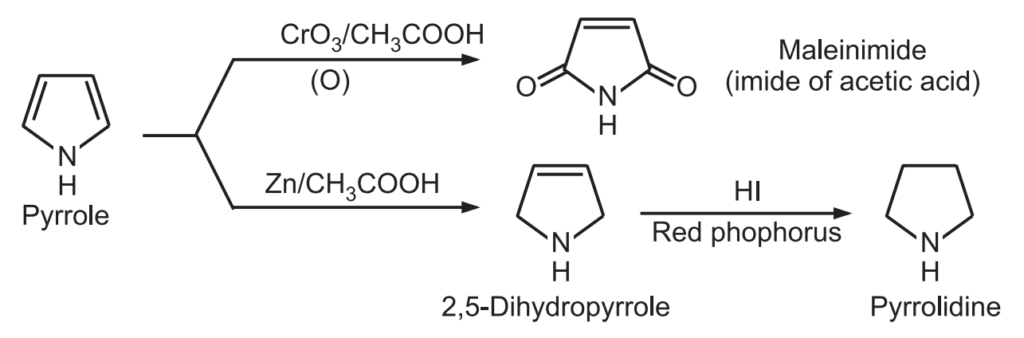
7. Oxidation and reduction: Pyrrole is oxidized to maleimide and on reduction it gives pyrrolidine.
Applications in Drug Synthesis
Pyrrole is a structural constituent of haem, chlorophyll, Vitamin B12, and bile pigments. Pyrrole ring is also present in the drug tolmetin (NSAID), ketorolac (NSAID), sunitinib (anti-cancer), ageliferin (anti-bacterial), elopiprazole (antipsychotic), procyclidine (an antimuscarinic drug to treat parkinsonism) and atorvastatin (lipid-lowering agent). Pyrrole is widely known as a biologically active scaffold having diversified therapeutic activities such as antipsychotic, β-adrenergic antagonist, anxiolytic, antibacterial, antifungal, antimalarial, and anticancer.
Make sure you also check our other amazing Article on : Pyrrole Synthesis